Should children aged 6 months through 4 years get the Pfizer COVID-19 vaccine?
This article steelmans the two main sides of this argument which we’ll call conventional and skeptical.
First, we’ll summarize the two sides in one sentence each to give a feeling of where we’re going. Next, we’ll list the main justifications. Finally, we’ll dive into the details and citations for those interested.
One-sentence summaries
- Conventional: For children aged 6 months through 4 years, COVID may cause serious medical issues including death, the Pfizer vaccine is likely effective in reducing COVID acquisition, its estimated benefits, including reducing community transmission, outweigh the estimated risks of side effects across the population, vaccination may have benefits in addition to natural immunity, vaccination in older groups is associated with reduced deaths, and the burden of proof is on those potentially jeopardizing their children and their community
- Skeptical: For children aged 6 months through 4 years that are healthy, risks of COVID-19 may be similar to the flu (influenza virus), the Pfizer vaccine is not likely very effective in reducing COVID acquisition and will likely wane quickly, it is in a new class of vaccines with limited safety data, community benefits are unclear, population-wide risk/benefit calculations do not account for individualized assessments and value judgments, natural immunity is likely robust, and the burden of proof for net benefits is on vaccine providers
Main justifications
-
Conventional:
- COVID is a serious risk for children aged 6 months through 4 years, including death, hospitalization, other diseases such as long COVID, COVID-associated myocarditis & pericarditis and multisystem inflammatory syndrome in children (MIS-C), it may increase disease burden on the community, and vaccination in older groups is associated with reduced deaths.
- Pfizer performed a randomized, double-blind, saline placebo controlled trial that showed the vaccine is likely at least somewhat effective and its side effects are likely similar to other vaccines.
- The vaccine may help in addition to any natural immunity and may reduce community transmission.
- Across the population, the estimated benefits outweigh the known, estimated risks.
- Given the above, the burden of proof is on those potentially jeopardizing their children and their communities, including contributing to community spread and hospital utilization.
-
Skeptical:
- COVID risks for children 6 months to 4 years may be similar to the flu (influenza virus).
- Getting the vaccine might make sense for those with underlying conditions associated with COVID hospitalization such as obesity, chronic lung disease, asthma, prematurity, neurologic disorder, feeding tube dependency, chronic metabolic disease, diabetes mellitus, blood disorders, sickle cell disease, cardiovascular disease, congenital heart disease, immunocompromised condition, and airway abnormality (Kim et al., 2020; Woodruff et al., 2022).
- If vaccinating, take extra care of the children to reduce exposure to COVID-19 between doses 1 and 2.
- Why was this Pfizer vaccine study underpowered? The 1954 Fields Trial for the polio vaccine was a randomized, double-blinded, placebo controlled trial with 750,000 children (Juskewitch et al., 2010) whereas the Pfizer study ended with just 1,106 children (U.S. FDA, 2022d; U.S. FDA, 2022e). Probably at least about 20% of parents of children 6 months to 4 years would have volunteered (U.S. CDC, 2022g) which is about 3.7 million children (U.S. CDC, 2022i). Cost shouldn’t be an issue because Pfizer made $36 billion on the vaccine in 2021 and projects $32 billion in 2022 (CNBC, 2022).
- Given this vaccine is in “a new class of vaccines” (U.S. FDA, 2022v) and, although rare, the similarly experimental Johnson & Johnson vaccine caused deaths from thrombosis with thrombocytopenia syndrome (U.S. CDC, 2022h), why have Pfizer studies been designed to be underpowered and unblinded shortly after the last dose so that long-term risks can’t be evaluated? There may be biologically plausible concerns about mRNA vaccines (Seneff et al., 2022; Föhse et al., 2021; Lee et al., 2021; Pfizer, 2021; Ndeupen et al., 2021; Igyártó et al., 2021) or production impurities (Krutzke et al., 2022), in addition to impacts of mRNA potentially persisting for two months after vaccination (Röltgen et al., 2022; Fertig et al., 2022) and spike protein potentially circulating four months after vaccination (Bansal et al., 2021), and potential spike protein toxicity (Rhea et al., 2021; Datta et al., 2021; Lei et al., 2021; Raghavan et al., 2021; Fernandes et al., 2022; Suzuki & Gychka, 2021; Pérez-Bermejo et al., 2020; Asandei et al., 2020; Buzhdygan et al., 2020; Biancatelli et al., 2021).
- Statistics and estimates across a population do not account for value judgments comparing known actions (vaccination) versus probabilistic events (acquiring a large dose of virus) given individualized risk and benefit estimates.
- Given the above, the burden of proof for net benefits, particularly for a new class of vaccines, is on the vaccine providers.
Details and citations
Deaths and hospitalizations
-
Conventional:
- For children through 4 years old in the U.S., COVID as the underlying cause of death (U.S. CDC, 2022j) is estimated to have killed 54 children in 2020, 145 in 2021 (provisional), and 71 in 2022 (provisional through June).
- Thousands of children per year through 4 years old in the U.S. have had COVID-19-associated hospitalization (U.S. CDC, 2022), with a relative surge during Omicron (Marks et al., 2022) that was comprised of 85% with COVID-19 as the primary reason for admission (Marks et al., 2022d), 63% with no underlying medical condition (Marks et al., 2022c), and 21% ended up in the Intensive Care Unit (ICU) (Marks et al., 2022b).
- Although only correlational, there is an association between vaccination and lower incidence of deaths in ages 5 and up (U.S. CDC, 2022m; Johnson et al., 2022):
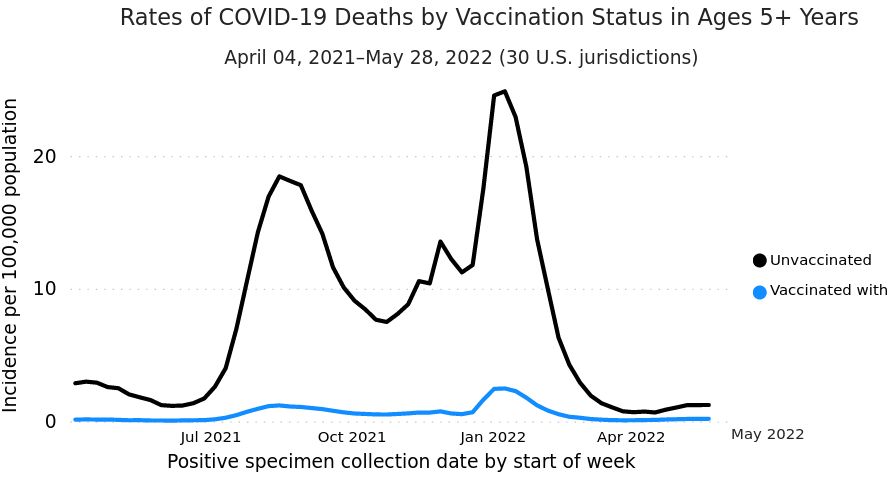
-
Skeptical:
- For children through 4 years old in the U.S., COVID-19 has killed fewer children than flu (influenza virus and pneumonia) as the underlying cause of death when comparing annualized rates for 2018-2019 to 2020-2022 (Flaxman et al., 2022; U.S. CDC, 2022j U.S. CDC, 2022k).
- The hospitalization rate of COVID for children through 4 years old has been less than flu (Delahoy et al., 2022).
Other potential risks
-
Conventional:
- Infecting others in the community (Schleiss et al., 2021)
- COVID-associated myocarditis (Rodriguez-Gonzalez et al., 2020) & pericarditis (Kermani-Alghoraishi et al., 2021)
- Long COVID (Wise, 2021)
- Multisystem inflammatory syndrome in children (MIS-C) (Chin et al., 2022; Feldstein et al., 2021; Levy et al., 2022)
- Acquiring diabetes (Barrett et al., 2022)
- Impact of sickness on families (e.g. work) (KFF, 2022)
-
Skeptical counter-arguments:
- Infecting others in the community (Ludvigsson, 2022; U.S. FDA, 2022aa)
- COVID-associated myocarditis & pericarditis (Tuvali et al., 2022)
- Long COVID (Zimmermann et al., 2022; Zimmermann et al., 2021; Al-Aly et al., 2021)
- Multisystem inflammatory syndrome in children (MIS-C) (Yalçinkaya et al., 2022)
Vaccine efficacy
Pfizer performed a randomized, double-blind, saline placebo controlled trial (U.S. FDA, 2022c)
-
Conventional:
- The vaccine effectiveness of avoiding COVID acquisition (at least 6 days after 3 doses):
- Children 6-23 months: 75.5% (95% confidence interval of -370.1% to 99.6%) (U.S. FDA, 2022d) with 1 case of COVID in the vaccine group (out of 277) versus 2 cases in the placebo group (out of 139).
- Children 2-4 years: 82.3% (95% confidence interval of -8.0% to 98.3%) (U.S. FDA, 2022e) with 2 cases of COVID in the vaccine group (out of 481) versus 5 cases in the placebo group (out of 209.
- Vaccine effectiveness is visualized in the cumulative incidence curves (U.S. FDA, 2022l) with the difference red line (placebo) showing higher COVID incidence than the blue line (vaccine); for example, for ages 2-4:
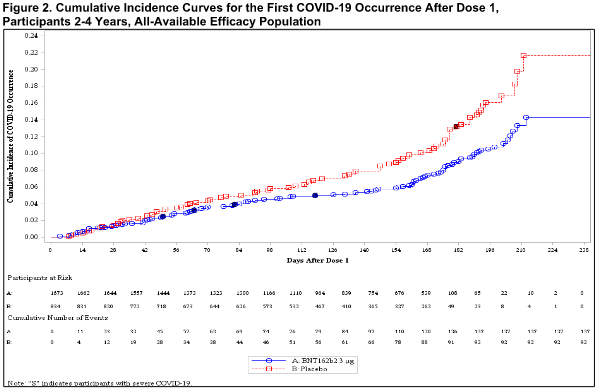
- The vaccine effectiveness of avoiding COVID acquisition (at least 6 days after 3 doses):
-
Skeptical:
- The results were statistically weak (95% confidence intervals crossed 0) as admitted in the Pfizer report (U.S. FDA, 2022o) and the FDA thought the interpretation of results may be overestimated (U.S. FDA, 2022s).
- The results are preliminary (U.S. FDA, 2022p) as the study did not achieve the protocol-specified 21 cases (10 total were observed) and the study is still on-going.
- The only hospitalization for severe COVID occurred in a vaccine recipient (U.S. FDA, 2022q) and 6 out of 8 cases of severe COVID (U.S. FDA, 2022x) were in the vaccine group, although the definition of severe COVID was non-standard (U.S. FDA, 2022ah) (i.e. including increased heart rate).
- Vaccine effectiveness is only inferred (U.S. FDA, 2022r) based on immunobridging (U.S. FDA, 2022ac) rather than clinically relevant endpoints like death, hospitalization, disease, etc. Immunobridging is comparing antibody levels to those of other age groups that did have clinically relevant endpoints evaluated and guessing that similar antibody levels will mean similar clinically relevant endpoints.
- The point estimates between dose 1 and dose 2 were negative (U.S. FDA, 2022ad; U.S. FDA, 2022ae) meaning that the vaccines might be associated with increased COVID as compared to the placebo group.
- The vaccine efficacy is likely to wane quickly (U.S. FDA, 2022t).
Adverse events
Monitored for up to 2 months (U.S. FDA, 2022k) after the last dose.
-
Conventional:
- Zero deaths (U.S. FDA, 2022)
- Zero potentially life-threatening events (U.S. FDA, 2022m) of grade 4 (U.S. FDA, 2007)
-
Skeptical:
- The FDA judged (Fleming-Dutra et al., 2022b) the “potential harms after vaccination” as “very low certainty […] because of the short duration of follow-up of 1 month after dose 3 and because only 31% of trial participants received dose 3, limiting the ability to detect serious adverse events that might occur at a higher rate after dose 3, and serious concern of imprecision because of the study size.”
- There were more severe adverse reactions of grade ≥ 3 (U.S. FDA, 2007) in the vaccine group than placebo: 4.3% vs. 3.6% (Fleming-Dutra et al., 2022).
- Some of the serious adverse events were considered by investigators and the FDA as potentially related to the vaccine (U.S. FDA, 2022g).
- Some patients were withdrawn (U.S. FDA, 2022h) after adverse events, some of which were considered by investigators and the FDA as potentially related to the vaccine.
- Secondary re-analyses of mRNA vaccine trials in adults shows concerning signals about serious adverse events (Fraiman et al., 2022).
Vaccination in addition to natural immunity
-
Conventional:
- In other age groups, for those that were already infected with the SARS-CoV-2 virus and created natural immunity, there is some evidence (Plumb et al., 2022) that vaccines decreased hospitalizations compared to the unvaccinated.
-
Skeptical:
- Natural immunity for children is now likely above 75% (U.S. FDA, 2022u).
- There is disagreement (Pugh et al., 2022) about the efficacy of natural immunity with and without vaccination.
- The measured antibodies in Pfizer’s study were lower (U.S. FDA, 2022w) against the current Omicron variant compared to previous variants meaning it might be less effective for newer variants.
Benefit/risk calculations
-
Conventional:
- The FDA and Pfizer estimate (U.S. FDA, 2022j) the benefits of vaccination for children 6 months to 4-5 years to be 5,880 fewer cases, 364 fewer hospitalizations, and 5 fewer deaths per million over 6 months.
- The FDA and Pfizer estimate (U.S. FDA, 2022j) the known risks of vaccination for children 6 months to 4-5 years to be 2.5 to 74.0 excess cases of myocarditis/pericarditis per million for males and 0.6 to 7.0 for females.
- Other pediatric vaccines have been approved for lower disease burdens (U.S. CDC, 2022l).
-
Skeptical:
- Myocarditis and pericarditis are rare but known risks associated with mRNA vaccines (Le Vu et al., 2022; U.S. FDA, 2022y; Chua et al., 2021; Buchan et al., 2022), although ~80% probably fully recover (U.S. CDC, 2022e). Blood was not sampled for troponin levels (Oster et al., 2022) to evaluate the potential of subclinical myocarditis (U.S. FDA, 2022ag).
- Anaphylaxis is a rare but known risk associated with mRNA vaccines (U.S. FDA, 2022z). It is particularly difficult (U.S. CDC, 2022c) to recognize in children.
- As noted by the FDA (U.S. FDA, 2022v), COVID-19 vaccines “represent a new class of vaccines […] based on new platform technologies”; in the case of this vaccine, rather than a classic vaccine of attenuated or live virus, it is modified mRNA in lipid nanoparticles that enter cells to stimulate protein production, so potential long-term risks are unknown.
- Death from vaccination is possible: the CDC determined at least nine deaths were caused by the Johnson & Johnson COVID-19 vaccine (U.S. CDC, 2022h) due to thrombosis with thrombocytopenia syndrome (TTS) (See et al., 2022), although this was an adenovirus-based DNA vaccine rather than mRNA.
- Some countries have tentatively paused vaccinations for some childhood age groups unless there are extenuating factors such as underlying disease, at-risk family members, etc.: Norway (for children < 15 years old) (Norwegian Institute of Public Health, 2022), Finland (for children < 12 years old) (Finnish Institute for Health and Welfare, 2022), Denmark (for children < 12 years old) (Danish Health Authority, 2022), and Sweden (for children < 12 years old) (Reuters, 2022).
- Participants will be unblinded (U.S. FDA, 2022af) 6 months after their last dose thus ending any randomized and controlled evaluation of long-term risks.
- It’s likely (U.S. FDA, 2022ab) that booster doses will be recommended thus increasing cumulative risks.
- Bell’s palsy is a rare but known risk associated with mRNA vaccines (U.S. CDC, 2022f).
- Guillain-Barré syndrome is a rare but known risk associated with mRNA vaccines (Hanson et al., 2022).
- Potential risks of antibody-dependent enhancement (ADE), original antigenic sin, vaccine-acquired immunodeficiency syndrome, etc. (Yamamoto, 2022)
- In children aged 5-11, between November 3 to February 27, 2022 (Hause et al., 2022), out of 7,578 reports of adverse events following vaccination submitted to the U.S. Vaccine Adverse Event Reporting System (VAERS), although only correlational (Rosenblum et al., 2022f), 194 were serious (2.6%), of which 4 were deaths (0.05%), and others included multisystem inflammatory syndrome in children (MIS-C), seizure, myocarditis, appendicitis, and allergic reaction. These numbers are out of about 16 million doses (Hause et al., 2022), although VAERS is subject to under-reporting and simulated reporting (Shimabukuro et al. 2015). Serious adverse events should be compared to normal background rates (Gubernot et al., 2021; Abara et al., 2022). Health impact reports were more common after the second dose (Hause et al., 2022b).
- In children aged 12-17, between December 14, 2020 to July 16, 2021 (Hause et al., 2021), out of 9,246 reports of adverse events following vaccination submitted to the U.S. Vaccine Adverse Event Reporting System (VAERS), although only correlational (Rosenblum et al., 2022f), 860 were serious (9.3%), of which 14 were deaths (0.15%), and others included myocarditis. These numbers are out of about 8.7 million doses (Hause et al., 2021), although VAERS is subject to under-reporting and simulated reporting (Shimabukuro et al. 2015). Serious adverse events should be compared to normal background rates (Gubernot et al., 2021; Abara et al., 2022).
- In adults, between December 2020 to June 2021 (Rosenblum et al., 2022b), out of 340,522 reports of adverse events following vaccination submitted to the U.S. Vaccine Adverse Event Reporting System (VAERS), although only correlational (Rosenblum et al., 2022f), 27,023 were serious (7.9%), of which 4,496 were deaths (1.3%), and others included COVID-19, coagulopathy, seizure, stroke, Bells’ palsy, anaphylaxis, myopericarditis, acute myocardial infarction, appendicitis, Guillain-Barré syndrome, multisystem inflammatory syndrome in adults, transverse myelitis, and narcolepsy. These numbers are out of about 298 million doses (Rosenblum et al., 2022c), although VAERS is subject to under-reporting and simulated reporting (Shimabukuro et al., 2015). Serious adverse events should be compared to normal background rates (Gubernot et al., 2021; Abara et al., 2022). Of the deaths with available death certificates, 46.5% were diseases of the heart (Rosenblum et al., 2022d). Health impact reports were more common after the second dose (Rosenblum et al., 2022e).
- Post-marketing observational studies and disproportionality analyses may have various limitations (Fraiman et al., 2022b).
References
126 references
- (Abara et al., 2022):
Abara, W. E., Gee, J., Delorey, M., Tun, Y., Mu, Y., Shay, D. K., & Shimabukuro, T. (2022). Expected Rates of Select Adverse Events After Immunization for Coronavirus Disease 2019 Vaccine Safety Monitoring. The Journal of infectious diseases, 225(9), 1569-1574. DOI: 10.1093/infdis/jiab628. https://doi.org/10.1093/infdis/jiab628
- (Al-Aly et al., 2021):
Al-Aly, Z., Bowe, B., & Xie, Y. (2021). Long Covid after Breakthrough COVID-19: the post-acute sequelae of breakthrough COVID-19. DOI: 10.21203/rs.3.rs-1062160/v1. https://doi.org/10.21203/rs.3.rs-1062160/v1
- (Asandei et al., 2020):
Asandei, A., Mereuta, L., Schiopu, I., Park, J., Seo, C. H., Park, Y., & Luchian, T. (2020). Non-receptor-mediated lipid membrane permeabilization by the SARS-CoV-2 spike protein S1 subunit. ACS Applied Materials & Interfaces, 12(50), 55649-55658. DOI: 10.1021/acsami.0c17044. https://doi.org/10.1021/acsami.0c17044
- (Bansal et al., 2021):
Bansal, S., Perincheri, S., Fleming, T., Poulson, C., Tiffany, B., Bremner, R. M., & Mohanakumar, T. (2021). Cutting edge: circulating exosomes with COVID spike protein are induced by BNT162b2 (Pfizer–BioNTech) vaccination prior to development of antibodies: a novel mechanism for immune activation by mRNA vaccines. The Journal of Immunology, 207(10), 2405-2410. DOI: 10.4049/jimmunol.2100637. https://doi.org/10.4049/jimmunol.2100637
- (Barrett et al., 2022):
Barrett, C. E., Koyama, A. K., Alvarez, P., Chow, W., Lundeen, E. A., Perrine, C. G., … & Saydah, S. (2022). Risk for newly diagnosed diabetes> 30 days after SARS-CoV-2 infection among persons aged< 18 years—United States, March 1, 2020–June 28, 2021. Morbidity and Mortality Weekly Report, 71(2), 59. DOI: 10.15585/mmwr.mm7102e2. https://doi.org/10.15585/mmwr.mm7102e2
- (Biancatelli et al., 2021):
Biancatelli, R. M. C., Solopov, P. A., Sharlow, E. R., Lazo, J. S., Marik, P. E., & Catravas, J. D. (2021). The SARS-CoV-2 spike protein subunit S1 induces COVID-19-like acute lung injury in Κ18-hACE2 transgenic mice and barrier dysfunction in human endothelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. DOI: 10.1152/ajplung.00223.2021. https://doi.org/10.1152/ajplung.00223.2021
- (Buchan et al., 2022):
Buchan, S. A., Seo, C. Y., Johnson, C., Alley, S., Kwong, J. C., Nasreen, S., … & Wilson, S. E. (2022). Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Network Open, 5(6), e2218505-e2218505. DOI: 10.1001/jamanetworkopen.2022.18505. https://doi.org/10.1001/jamanetworkopen.2022.18505
- (Buzhdygan et al., 2020):
Buzhdygan, T. P., DeOre, B. J., Baldwin-Leclair, A., Bullock, T. A., McGary, H. M., Khan, J. A., … & Ramirez, S. H. (2020). The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiology of disease, 146, 105131. DOI: 10.1016/j.nbd.2020.105131. https://doi.org/10.1016/j.nbd.2020.105131
- (Chin et al., 2022):
Chin, S. E., Bhavsar, S. M., Corson, A., Ghersin, Z. J., & Kim, H. S. (2022). Cardiac Complications Associated with COVID-19, MIS-C, and mRNA COVID-19 Vaccination. Pediatric Cardiology, 1-6. DOI: 10.1007/s00246-022-02851-x. https://doi.org/10.1007/s00246-022-02851-x
- (Chua et al., 2021):
Chua, G. T., Kwan, M. Y. W., Chui, C. S., Smith, R. D., Cheung, E. C. L., Tian, T., … & Ip, P. (2021). Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clinical Infectious Diseases. DOI: 10.1093/cid/ciab989. https://doi.org/10.1093/cid/ciab989
- (CNBC, 2022):
“Pfizer expects $32 billion in Covid vaccine sales for 2022 […] Pfizer sold $36.7 billion of its Covid vaccine worldwide in 2021, representing 45% of its total year revenue of $81.2 billion.”
CNBC (2022). What’s next for Pfizer, Moderna beyond their projected $51 billion in combined Covid vaccine sales this year. Retrieved July, 2022, from https://www.cnbc.com/2022/03/03/covid-pfizer-moderna-project-51-billion-in-combined-vaccine-sales-this-year.html
- (Danish Health Authority, 2022):
“Due to the high level of immunity in the population and signs showing that the curve for the third wave is flattening, combined with the knowledge of infection rates normally declines with the seasons changing, the Danish Health Authority has decided to phase out the current vaccination program for all age groups - including the program for 5-11 year-olds.”
Danish Health Authority (2022). No further plans of extensions to the covid-19 vaccination program. Retrieved July, 2022, from https://sst.dk/en/English/News/2022/No-further-plans-of-extensions-to-the-covid-19-vaccination-program
- (Datta et al., 2021):
Datta, G., Miller, N. M., Halcrow, P. W., Khan, N., Colwell, T., Geiger, J. D., & Chen, X. (2021). SARS-CoV-2 S1 Protein Induces Endolysosome Dysfunction and Neuritic Dystrophy. Frontiers in Cellular Neuroscience, 441. DOI: 10.3389/fncel.2021.777738. https://doi.org/10.3389/fncel.2021.777738
- (Delahoy et al., 2022):
“Among children 0–4 years old, influenza-associated hospitalization rates for the 2017–18 season (71.0) and 2018-19 season (70.9) were similar to the annual COVID-19-associated hospitalization rate (66.8), but the influenza-associated hospitalization rate for the 2019-20 season (91.5) was higher. […] Figure 2. Cumulative influenza- and COVID-19-associated hospitalization rates per 100,000 children <18 years old, by age group – FluSurv-NET1 and COVID-NET2, 2017–2021”
Delahoy, M., Ujamaa, D., Taylor, C. A., Cummings, C., Anglin, O., Holstein, R. A., … & Garg, S. (2022). Comparison of influenza and COVID-19-associated hospitalizations among children< 18 years old in the United States-FluSurv-NET (October-April 2017-2021) and COVID-NET (October 2020-September 2021). medRxiv. DOI: 10.1101/2022.03.09.22271788. https://doi.org/10.1101/2022.03.09.22271788 ; Recommended: https://www.medrxiv.org/content/10.1101/2022.03.09.22271788v1.full.pdf#page=8
- (Feldstein et al., 2021):
Feldstein, L. R., Tenforde, M. W., Friedman, K. G., Newhams, M., Rose, E. B., Dapul, H., … & Overcoming COVID-19 Investigators. (2021). Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA, 325(11), 1074-1087. DOI: 10.1001/jama.2021.2091. https://doi.org/10.1001/jama.2021.2091
- (Fernandes et al., 2022):
Fernandes, B. H. V., Feitosa, N. M., Barbosa, A. P., Bomfim, C. G., Garnique, A. M., Rosa, I. F., … & Charlie-Silva, I. (2022). Toxicity of spike fragments SARS-CoV-2 S protein for zebrafish: A tool to study its hazardous for human health?. Science of The Total Environment, 813, 152345. DOI: 10.1016/j.scitotenv.2021.152345. https://doi.org/10.1016/j.scitotenv.2021.152345
- (Fertig et al., 2022):
Fertig, T. E., Chitoiu, L., Marta, D. S., Ionescu, V. S., Cismasiu, V. B., Radu, E., … & Gherghiceanu, M. (2022). Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines, 10(7), 1538. DOI: 10.3390/biomedicines10071538. https://doi.org/10.3390/biomedicines10071538 ; Recommended: https://mdpi-res.com/d_attachment/biomedicines/biomedicines-10-01538/article_deploy/biomedicines-10-01538-v4.pdf
- (Finnish Institute for Health and Welfare, 2022):
“to children aged 5 to 11 in risk groups, whose illness or condition may predispose them to severe coronavirus disease; to children aged 5 to 11 who are close to severely immunodeficient people.”
Finnish institute for health and welfare (2022). Coronavirus vaccinations for children and young people. Retrieved July, 2022, from https://thl.fi/en/web/infectious-diseases-and-vaccinations/what-s-new/coronavirus-covid-19-latest-updates/vaccines-and-coronavirus/coronavirus-vaccinations-for-children-and-young-people
- (Flaxman et al., 2022):
“Table 1(a) Age: < 1 year […] #Influenza and pneumonia (J09-J18): 4.1 […] #COVID-19 (U07.1): 3.5
Table 1(b) Age: 1-4 year olds […] #Influenza and pneumonia (J09-J18): 0.8 […] #COVID-19 (U07.1): 0.5” (Pages 5-6)
Flaxman, S., Whittaker, C., Semenova, E., Rashid, T., Parks, R., Blenkinsop, A., … & Ratmann, O. (2022). Covid-19 is a leading cause of death in children and young people ages 0-19 years in the United States. medRxiv. DOI: 10.1101/2022.05.23.22275458. https://doi.org/10.1101/2022.05.23.22275458 ; Recommended: https://www.medrxiv.org/content/10.1101/2022.05.23.22275458v3.full.pdf#page=5
- (Fleming-Dutra et al., 2022):
“Severe local and systemic adverse reactions (grade 3 or higher, defined as interfering with daily activity) occurred in 4.3% and 3.6% of vaccine recipients and placebo recipients, respectively.” (Page 4)
Fleming-Dutra, K. E., Wallace, M., Moulia, D. L., Twentyman, E., Roper, L. E., Hall, E., … & Oliver, S. E. (2022). Interim recommendations of the advisory committee on immunization practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. DOI: 10.15585/mmwr.mm7126e2. https://doi.org/10.15585/mmwr.mm7126e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7126e2-H.pdf#page=4
- (Fleming-Dutra et al., 2022b):
Fleming-Dutra, K. E., Wallace, M., Moulia, D. L., Twentyman, E., Roper, L. E., Hall, E., … & Oliver, S. E. (2022b). Interim recommendations of the advisory committee on immunization practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. DOI: 10.15585/mmwr.mm7126e2. https://doi.org/10.15585/mmwr.mm7126e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7126e2-H.pdf#page=4
- (Fohse et al., 2021):
Föhse, F. K., Geckin, B., Overheul, G. J., van de Maat, J., Kilic, G., Bulut, O., … & Netea, M. G. (2021). The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses. MedRxiv. DOI: 10.1101/2021.05.03.21256520. https://doi.org/10.1101/2021.05.03.21256520 ; Recommended: https://www.medrxiv.org/content/10.1101/2021.05.03.21256520v1.full.pdf
- (Fraiman et al., 2022):
“The results show an excess risk of serious AESIs greater than the reduction in COVID-19 hospitalizations in both Pfizer and Moderna trials. These results are compatible with a recent preprint analysis of COVID-19 vaccine trials by Benn et al., which found no evidence of a reduction in overall mortality in the mRNA vaccine trials based on data from the later, March 2021 BLA (Biologics License Application) timepoints that underpinned subsequent regulatory approval (31 deaths in the vaccine arms versus 30 events in the placebo arms; RR 1.03, 95% CI 0.63 to 1.71).28 Our analysis as well as Benn et al. point to the need for formal harm-benefit analyses especially in individuals at low risk of COVID-19 hospitalization or death.” (Page 9)
Fraiman, J., Erviti, J., Jones, M., Greenland, S., Whelan, P., Kaplan, R. M., & Doshi, P. (2022). Serious Adverse Events of Special Interest Following mRNA Vaccination in Randomized Trials. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4125239
- (Fraiman et al., 2022b):
“Despite the unprecedented scale of COVID-19 vaccine administration, the AESI types identified in our study may still be challenging to detect with observational methods. Most cohort study designs crucially depend upon comparing the risks of adverse events “observed” against a background (or “expected”) risk. However, background incidence risks display great variation, by database, age group, and sex.30 If the risk ratio of 1.4 estimated in our analysis were the actual effect size, it could be quite difficult to unambiguously replicate it with observational data given concerns about systematic as well as random errors. In addition, disproportionality analyses following COVID-19 vaccination also have limitations, particularly with respect to the type of adverse events seen in our study. The majority of SAE types that contributed to our results are relatively common events, such as ischemic stroke, acute coronary syndrome, and brain hemorrhage. This complicates signal detection because clinical suspicion of an adverse vaccine reaction following an event commonly seen in clinical practice will be lower than for less commonly observed SAEs like myocarditis. For this reason, the basic ingredient for effective pharmacovigilance–clinical suspicion leading to the filing of an individual case safety report–may be far less common in the post-authorization setting.” (Page 9)
Fraiman, J., Erviti, J., Jones, M., Greenland, S., Whelan, P., Kaplan, R. M., & Doshi, P. (2022b). Serious Adverse Events of Special Interest Following mRNA Vaccination in Randomized Trials. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4125239
- (Gubernot et al., 2021):
Gubernot, D., Jazwa, A., Niu, M., Baumblatt, J., Gee, J., Moro, P., … & Bennett, S. (2021). US Population-Based background incidence rates of medical conditions for use in safety assessment of COVID-19 vaccines. Vaccine, 39(28), 3666-3677. DOI: 10.1016/j.vaccine.2021.05.016. https://doi.org/10.1016/j.vaccine.2021.05.016 ; Recommended: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8118666/pdf/main.pdf
- (Hanson et al., 2022):
Hanson, K. E., Goddard, K., Lewis, N., Fireman, B., Myers, T. R., Bakshi, N., … & Klein, N. P. (2022). Incidence of Guillain-Barré Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. JAMA network open, 5(4), e228879-e228879. DOI: 10.1001/jamanetworkopen.2022.8879. https://doi.org/10.1001/jamanetworkopen.2022.8879
- (Hause et al., 2021):
“As of July 16, 2021, approximately 8.9 million U.S. adolescents aged 12–17 years had received Pfizer-BioNTech vaccine. VAERS received 9,246 reports after Pfizer-BioNTech vaccination in this age group; 90.7% of these were for nonserious adverse events and 9.3% were for serious adverse events, including myocarditis (4.3%). […] CDC reviewed 14 reports of death after vaccination. Among the decedents, four were aged 12–15 years and 10 were aged 16–17 years. All death reports were reviewed by CDC physicians; impressions regarding cause of death were pulmonary embolism (two), suicide (two), intracranial hemorrhage (two), heart failure (one), hemophagocytic lymphohistiocytosis and disseminated Mycobacterium chelonae infection (one), and unknown or pending further records (six). […] TABLE 2. Most frequent symptoms, signs, diagnostic results, and conditions reported to the Vaccine Adverse Event Reporting System for adolescents aged 12–17 years after receipt of the Pfizer-BioNTech COVID-19 vaccine (N = 9,246) — United States, December 14, 2020–July 16, 2021” (Pages 1, 3)
Hause, A. M., Gee, J., Baggs, J., Abara, W. E., Marquez, P., Thompson, D., … & Shay, D. K. (2021b). COVID-19 vaccine safety in adolescents aged 12–17 years—United States, December 14, 2020–July 16, 2021. Morbidity and Mortality Weekly Report, 70(31), 1053. DOI: 10.15585/mmwr.mm7031e1. https://doi.org/10.15585/mmwr.mm7031e1 ; Recommended: https://www.cdc.gov/mmwr/volumes/70/wr/pdfs/mm7031e1-H.pdf
- (Hause et al., 2022):
“As of February 27, 2022, >16 million BNT-162b2 vaccine doses had been administered to children ages 5–11 years. […] During November 3, 2021–February 27, 2022, VAERS received and processed 7,578 reports of adverse events for children ages 5–11 years who received BNT-162b2 vaccine […] Among the 194 serious reports to VAERS, the most common clinical impressions included MIS-C (26; 13.4%), seizure (21; 10.8%), myocarditis (19; 9.7%), appendicitis (13; 6.7%), and allergic reaction (8; 4.1%). […] VAERS received four reports of death after vaccination during the analytic period. Two occurred in females, ages 5 and 6 years, who had complex medical histories, including autonomic instability and frequent admissions to pediatric intensive care; autopsy was not performed for either decedent. One death occurred in a female aged 7 years; upon autopsy, evidence of influenza infection was documented. One death occurred in an 8-year-old male, 8 days after dose 2. He experienced nausea and vomiting the night before his death; an autopsy was performed and results are pending. The available information for these reports did not support a causal association between vaccination and death for any of the decedents.”
Hause, A. M., Shay, D. K., Klein, N. P., Abara, W. E., Baggs, J., Cortese, M. M., … & Shimabukuro, T. T. (2022). Safety of COVID-19 Vaccination in US Children Ages 5–11 Years. Pediatrics. DOI: 10.1542/peds.2022-057313. https://doi.org/10.1542/peds.2022-057313
- (Hause et al., 2022b):
“systemic reactions were more frequently reported in the week after dose 2 (16,161; 41.0%) than dose 1 (17,214; 35.3%). For both doses, reactions were most frequently reported the day after vaccination.”
Hause, A. M., Shay, D. K., Klein, N. P., Abara, W. E., Baggs, J., Cortese, M. M., … & Shimabukuro, T. T. (2022b). Safety of COVID-19 Vaccination in US Children Ages 5–11 Years. Pediatrics. DOI: 10.1542/peds.2022-057313. https://doi.org/10.1542/peds.2022-057313
- (Igyarto et al., 2021):
Igyártó, B. Z., Jacobsen, S., & Ndeupen, S. (2021). Future considerations for the mRNA-lipid nanoparticle vaccine platform. Current Opinion in Virology, 48, 65-72. DOI: 10.1016/j.coviro.2021.03.008. https://doi.org/10.1016/j.coviro.2021.03.008
- (Johnson et al., 2022):
“FIGURE. Weekly trends in age-standardized incidence of COVID-19 cases (April 4–December 25, 2021) and deaths (April 4–December 4, 2021) for unvaccinated compared with fully vaccinated persons, overall and by receipt of booster doses and national weighted estimates of variant proportions — 25 U.S. jurisdictions” (Page 6)
Johnson, A. G., Amin, A., Ali, A., Hoots, B., Cadwell, B., Arora, S., Avoundjian, T., Awofeso, A., Barnes, J., Bayoumi, N., Busen, K., Chang, C., Cima, M., Crockett, M., Cronquist, A., Davidson, S., Davis, E., Delgadillo, J., Dorabawila, V., …, Scobie, H. (2022). COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 US Jurisdictions, April 4–December 25, 2021. MMWR. Morbidity and mortality weekly report, 71. DOI: 10.15585/mmwr.mm7104e2. https://doi.org/10.15585/mmwr.mm7104e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7104e2-H.pdf#page=6
- (Juskewitch et al., 2010):
“a placebo-controlled trial was conducted at the same time in a smaller group of 750,000 children. Students from the 1st, 2nd, and 3rd grades in these regions were all consented for injection in this double-blind study. Half of these consented students were given the polio vaccine and the other half received a placebo injection in an alternating fashion.” (Page 2)
Juskewitch, BA, J. E., Tapia, BA, C. J., & Windebank, A. J. (2010). Lessons from the Salk polio vaccine: methods for and risks of rapid translation. Clinical and translational science, 3(4), 182-185. DOI: 10.1111/j.1752-8062.2010.00205.x. https://doi.org/10.1111/j.1752-8062.2010.00205.x
- (Kermani-Alghoraishi et al., 2021):
Kermani-Alghoraishi, M., Pouramini, A., Kafi, F., & Khosravi, A. (2021). Coronavirus disease 2019 (COVID-19) and severe pericardial effusion: from pathogenesis to management: a case report based systematic review. Current Problems in Cardiology, 100933. DOI: 10.1016/j.cpcardiol.2021.100933. https://doi.org/10.1016/j.cpcardiol.2021.100933
- (KFF, 2022):
“Percent of parents who say in the past year, they or another adult in their household left a job or changed work schedules in order to take care of their children: […] Parent to child under age 5: 48%”
KFF (2022). KFF COVID-19 vaccine monitor: The impact of the coronavirus pandemic on the wellbeing Of parents and children. Retrieved July, 2022, from https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-the-impact-of-the-coronavirus-pandemic-on-the-wellbeing-of-parents-and-children/
- (Kim et al., 2020):
Kim, L., Whitaker, M., O’Halloran, A., Kambhampati, A., Chai, S. J., Reingold, A., … & COVID-NET Surveillance Team. (2020). Hospitalization rates and characteristics of children aged< 18 years hospitalized with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 1–July 25, 2020. Morbidity and Mortality Weekly Report, 69(32), 1081. DOI: 10.15585/mmwr.mm6932e3. https://doi.org/10.15585/mmwr.mm6932e3
- (Krutzke et al., 2022):
Krutzke, L., Rösler, R., Allmendinger, E., Engler, T., Wiese, S., & Kochanek, S. (2022). Process-and product-related impurities in the ChAdOx1 nCov-19 vaccine. Elife, 11, e78513. DOI: 10.7554/eLife.78513. https://doi.org/10.7554/eLife.78513 ; Recommended: https://elifesciences.org/articles/78513.pdf
- (Le Vu et al., 2022):
Le Vu, S., Bertrand, M., Jabagi, M. J., Botton, J., Drouin, J., Baricault, B., … & Zureik, M. (2022). Age and sex-specific risks of myocarditis and pericarditis following Covid-19 messenger RNA vaccines. Nature communications, 13(1), 1-9. DOI: 10.1038/s41467-022-31401-5. https://doi.org/10.1038/s41467-022-31401-5
- (Lee et al., 2021):
Lee, E. J., Cines, D. B., Gernsheimer, T., Kessler, C., Michel, M., Tarantino, M. D., … & Bussel, J. B. (2021). Thrombocytopenia following pfizer and moderna SARS‐CoV‐2 vaccination. American journal of hematology. DOI: 10.1002/ajh.26132. https://doi.org/10.1002/ajh.26132 ; Recommended: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8014568/pdf/AJH-96-534.pdf
- (Lei et al., 2021):
Lei, Y., Zhang, J., Schiavon, C. R., He, M., Chen, L., Shen, H., … & Shyy, J. Y. (2021). SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circulation research, 128(9), 1323-1326. DOI: 10.1161/CIRCRESAHA.121.318902. https://doi.org/10.1161/CIRCRESAHA.121.318902
- (Levy et al., 2022):
Levy, M., Recher, M., Hubert, H., Javouhey, E., Fléchelles, O., Leteurtre, S., & Angoulvant, F. (2022). Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. Jama, 327(3), 281-283. DOI: 10.1001/jama.2021.23262. https://doi.org/10.1001/jama.2021.23262
- (Ludvigsson, 2022):
Ludvigsson, J. F. (2020). Children are unlikely to be the main drivers of the COVID‐19 pandemic–a systematic review. Acta Paediatrica, 109(8), 1525-1530. DOI: 10.1111/apa.15371. https://doi.org/10.1111/apa.15371
- (Marks et al., 2022):
“FIGURE. COVID-19–associated hospitalization rates among infants and children aged 0–4 years, by age group (3-week moving average) — Coronavirus Disease 2019–Associated Hospitalization Surveillance Network, 14 states, March 2020–February 2022” (Page 3)
Marks, K. J., Whitaker, M., Agathis, N. T., Anglin, O., Milucky, J., Patel, K., … & COVID-NET Surveillance Team. (2022). Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. Morbidity and Mortality Weekly Report, 71(11), 429. DOI: 10.15585/mmwr.mm7111e2. https://doi.org/10.15585/mmwr.mm7111e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7111e2-H.pdf#page=3
- (Marks et al., 2022b):
“Compared with Delta predominance, hospital length of stay during Omicron predominance was shorter (2 versus 1.5 days, p = 0.002) and the proportion of hospitalized infants and children requiring ICU admission (27% versus 21%, p = 0.02) was lower.” (Page 2)
Marks, K. J., Whitaker, M., Agathis, N. T., Anglin, O., Milucky, J., Patel, K., … & COVID-NET Surveillance Team. (2022b). Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. Morbidity and Mortality Weekly Report, 71(11), 429. DOI: 10.15585/mmwr.mm7111e2. https://doi.org/10.15585/mmwr.mm7111e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7111e2-H.pdf#page=2
- (Marks et al., 2022c):
“During Omicron predominance, 37% of hospitalized infants and children had one or more underlying medical condition.” (Page 2)
Marks, K. J., Whitaker, M., Agathis, N. T., Anglin, O., Milucky, J., Patel, K., … & COVID-NET Surveillance Team. (2022c). Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. Morbidity and Mortality Weekly Report, 71(11), 429. DOI: 10.15585/mmwr.mm7111e2. https://doi.org/10.15585/mmwr.mm7111e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7111e2-H.pdf#page=2
- (Marks et al., 2022d):
“Although there was some variation across periods, most patients had COVID-19–related symptoms recorded at admission (87%) and COVID-19 as the primary reason for admission (85%).” (Page 2)
Marks, K. J., Whitaker, M., Agathis, N. T., Anglin, O., Milucky, J., Patel, K., … & COVID-NET Surveillance Team. (2022d). Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19—COVID-NET, 14 States, March 2020–February 2022. Morbidity and Mortality Weekly Report, 71(11), 429. DOI: 10.15585/mmwr.mm7111e2. https://doi.org/10.15585/mmwr.mm7111e2 ; Recommended: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7111e2-H.pdf#page=2
- (Ndeupen et al., 2021):
Ndeupen, S., Qin, Z., Jacobsen, S., Bouteau, A., Estanbouli, H., & Igyártó, B. Z. (2021). The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience, 24(12), 103479. DOI: 10.1016/j.isci.2021.103479. https://doi.org/10.1016/j.isci.2021.103479
- (Norwegian Institute of Public Health, 2022):
“Children and adolescents 5–15 years with severe underlying disease […] those born in 2017 who have reached 5 years […] Can be vaccinated with both 1 and 2 doses if they and their parents wish. Vaccine is particularly relevant for: Children with chronic diseases; Families where children have close contact with people with particular need for protection; Children who have an increased risk because they will move to or stay in countries with a higher risk of transmission or poorer access to health services than in Norway, or children who for other reasons live in a very vulnerable situation; For those who choose the vaccine, the NIPH considers that one dose provides the best benefit-disadvantage balance in this age group.”
Norwegian Institute of Public Health (2022). Coronavirus vaccine information for the public. Retrieved July, 2022, from https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/#vaccination-of-children-and-adolescents
- (Oster et al., 2022):
Oster, M. E., Shay, D. K., Su, J. R., Gee, J., Creech, C. B., Broder, K. R., … & Shimabukuro, T. T. (2022). Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. Jama, 327(4), 331-340. DOI: 10.1001/jama.2021.24110. https://doi.org/10.1001/jama.2021.24110
- (Perez-Bermejo et al., 2020):
Pérez-Bermejo, J. A., Kang, S., Rockwood, S. J., Simoneau, C. R., Joy, D. A., Ramadoss, G. N., … & McDevitt, T. C. (2020). SARS-CoV-2 infection of human iPSC-derived cardiac cells predicts novel cytopathic features in hearts of COVID-19 patients. BioRxiv. DOI: 10.1101%2F2020.08.25.265561. https://doi.org/10.1101%2F2020.08.25.265561
- (Pfizer, 2021):
“Mean (Sexes-Combined) Concentration and Recovery of Total Radioactivity in Whole Blood, Plasma and Tissues Following Single Intramuscular Administration of [3 H]-08-A01-C01 to Wistar Han Rats” (Pages 23-26)
Pfizer. (2022). Tissue distribution study of a [3H]-labelled lipid nanoparticle-mRNA formulation containing ALC-0315 and ALC-0159 following intramuscular administration in Wistar Han rats. Retrieved July, 2022, from https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M4_4223_185350.pdf#page=23
- (Pfizer, 2022):
“Serious and unexpected side effects may occur. The possible side effects of the vaccine are still being studied in clinical trials.” (Page 4)
Pfizer. (2022). Fact sheet for recipients and caregivers about the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19) for use in individuals 6 months through 4 years of age. Retrieved July, 2022, from https://www.fda.gov/media/159313/download https://www.fda.gov/media/159313/download#page=4
- (Plumb et al., 2022):
Plumb, I. D., Feldstein, L. R., Barkley, E., Posner, A. B., Bregman, H. S., Hagen, M. B., & Gerhart, J. L. (2022). Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19–associated hospitalization among adults with previous SARS-CoV-2 infection—United States, June 2021–February 2022. Morbidity and Mortality Weekly Report, 71(15), 549. DOI: 10.15585/mmwr.mm7115e2. https://doi.org/10.15585/mmwr.mm7115e2
- (Pugh et al., 2022):
“Data also suggested that the antibodies elicited by vaccination have less potency and breadth than those generated by natural infection, although the overall neutralising potency of plasma is greater following vaccination. […] although data suggest that vaccination is still beneficial in those with natural immunity, and indeed may achieve the maximal level of protection, data suggest that the absolute reduction in risk that vaccination achieves in those with natural immunity may be small. One large study found that 767 individuals with natural immunity needed to be vaccinated to prevent one reinfection during follow-up. […] an early rapid analysis from the SIREN study suggests that protection against infection afforded by natural immunity may be at least as good as that afforded by the two vaccine doses that are deemed sufficient to satisfy vaccine requirements in many jurisdictions. The rapid analysis suggests that the rate of Omicron in those who had received two vaccinations was 73.4 infections (per 10 000 person days), compared with only 60.9 infections (per 10 000 person days) in those who were unvaccinated but had evidence of prior infection. Notably though, this fell to 41.6 per 10 000 in those who had received three vaccine doses. There are still significant gaps in our understanding of the respective strength and durability of natural and vaccine-induced immunity, as well as the implications that the Omicron variant (and future virus variants) may have. […] on the basis of existing data, it is plausible that naturally acquired immunity may be as good as the degree of vaccine-mediated immunity” (Page 3)
Pugh, J., Savulescu, J., Brown, R. C., & Wilkinson, D. (2022). The unnaturalistic fallacy: COVID-19 vaccine mandates should not discriminate against natural immunity. Journal of medical ethics, 48(6), 371-377. http://doi.org/10.1136/medethics-2021-107956 ; Recommended: https://jme.bmj.com/content/medethics/48/6/371.full.pdf#page=3
- (Raghavan et al., 2021):
Raghavan, S., Kenchappa, D. B., & Leo, M. D. (2021). SARS-CoV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity. Frontiers in cardiovascular medicine, 582. DOI: 10.3389/fcvm.2021.687783. https://doi.org/10.3389/fcvm.2021.687783
- (Rhea et al., 2021):
Rhea, E. M., Logsdon, A. F., Hansen, K. M., Williams, L. M., Reed, M. J., Baumann, K. K., … & Erickson, M. A. (2021). The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nature neuroscience, 24(3), 368-378. DOI: 10.1038/s41593-020-00771-8. https://doi.org/10.1038/s41593-020-00771-8
- (Reuters, 2022):
“Sweden has decided against recommending COVID vaccines for kids aged 5-11, the Health Agency said on Thursday, arguing that the benefits did not outweigh the risks.”
Reuters (2022). Sweden decides against recommending COVID vaccines for kids aged 5-11. Retrieved July, 2022, from https://www.reuters.com/world/europe/sweden-decides-against-recommending-covid-vaccines-kids-aged-5-12-2022-01-27/
- (Rodriguez-Gonzalez et al., 2020):
Rodriguez-Gonzalez, M., Castellano-Martinez, A., Cascales-Poyatos, H. M., & Perez-Reviriego, A. A. (2020). Cardiovascular impact of COVID-19 with a focus on children: A systematic review. World journal of clinical cases, 8(21), 5250. DOI: 10.12998/wjcc.v8.i21.5250. https://doi.org/10.12998/wjcc.v8.i21.5250
- (Roltgen et al., 2022):
Röltgen, K., Nielsen, S. C., Silva, O., Younes, S. F., Zaslavsky, M., Costales, C., … & Boyd, S. D. (2022). Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell, 185(6), 1025-1040. DOI: 10.1016/j.cell.2022.01.018. https://doi.org/10.1016/j.cell.2022.01.018
- (Rosenblum et al., 2022):
“Table 5: Local and systemic reactions and health impacts following mRNA COVID-19 vaccines reported during days 0–7 after vaccination to v-safe, by manufacturer and dose […] Reported medical care: [Dose one:] 0.8% […] [Dose two:] 0.9%” (Page 7)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=7. https://doi.org/10.1016/S1473-3099(22)00054-8#page=7 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=7
- (Rosenblum et al., 2022b):
“Table 2: Frequency and rates of adverse events of special interest reported to VAERS by recipients of mRNA COVID-19 vaccines” (Page 5)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022b). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=5. https://doi.org/10.1016/S1473-3099(22)00054-8#page=5 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=5
- (Rosenblum et al., 2022c):
“From Dec 14, 2020, to June 14, 2021, 298,792,852 doses of mRNA COVID-19 vaccines were administered in the USA” (Page 3)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022c). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=3. https://doi.org/10.1016/S1473-3099(22)00054-8#page=3 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=3
- (Rosenblum et al., 2022d):
“Death certificates or autopsy reports were available for clinical review for 808 (18·1%) of 4471 reports of deaths. Among these, causes of death were most commonly diseases of the heart (376 [46·5%]) and COVID-19 (102 [12·6%]; appendix pp 3–4).” (Page 4)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022d). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=4. https://doi.org/10.1016/S1473-3099(22)00054-8#page=4 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=4
- (Rosenblum et al., 2022e):
“Reported health impacts were greater following dose two of either vaccine than dose one” (Page 6)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022e). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=6. https://doi.org/10.1016/S1473-3099(22)00054-8#page=6 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=6
- (Rosenblum et al., 2022f):
“VAERS is designed as an early warning system to detect potential safety signals, and VAERS data alone generally cannot establish causal relationships between vaccination and adverse events […] Additionally, VAERS reports require interpretation to identify whether reports meet clinical case definitions.” (Page 9)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022f). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=9. https://doi.org/10.1016/S1473-3099(22)00054-8#page=9 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=9
- (Rosenblum et al., 2022g):
“An important limitation of this report is one shared by all VAERS analyses: we used data from a passive reporting system subject to underreporting and variable or incomplete reporting.” (Page 9)
Rosenblum, H. G., Gee, J., Liu, R., Marquez, P. L., Zhang, B., Strid, P., … & Shay, D. K. (2022g). Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. The Lancet Infectious Diseases. DOI: 10.1016/S1473-3099(22)00054-8#page=9. https://doi.org/10.1016/S1473-3099(22)00054-8#page=9 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S1473-3099%2822%2900054-8#page=9
- (Schleiss et al., 2021):
Schleiss, M. R., John, C. C., & Permar, S. R. (2021). Children are the key to the Endgame: A case for routine pediatric COVID vaccination. Vaccine, 39(38), 5333. DOI: 10.1016/j.vaccine.2021.08.005. https://doi.org/10.1016/j.vaccine.2021.08.005
- (See et al., 2022):
“All deaths associated with confirmed TTS cases after Ad26.COV2.S vaccination (that is, 8 with vaccination by 31 August 2021 and 1 with vaccination after 31 August 2021) occurred in patients with cerebral hemorrhage; we also noted 2 additional deaths involving cerebral hemorrhage after Ad26.COV2.S vaccination but without documented thrombosis where the clinical picture was otherwise compatible with TTS.” (Page 8)
See, I., Lale, A., Marquez, P., Streiff, M. B., Wheeler, A. P., Tepper, N. K., … & Shay, D. K. (2022). Case Series of Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination—United States, December 2020 to August 2021. Annals of internal medicine, 175(4), 513-522. DOI: 10.7326/M21-4502. https://doi.org/10.7326/M21-4502 ; Recommended: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8787833/pdf/aim-olf-M214502.pdf#page=8
- (Seneff et al., 2022):
Seneff, S., Nigh, G., Kyriakopoulos, A. M., & McCullough, P. A. (2022). Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and Chemical Toxicology, 164, 113008. DOI: 10.1016/j.fct.2022.113008. https://doi.org/10.1016/j.fct.2022.113008
- (Shimabukuro et al. 2015):
“Like all spontaneous public health reporting systems, VAERS has limitations [1,14]. VAERS is subject to reporting bias, including underreporting of adverse events – especially common, mild ones – and stimulated reporting, which is elevated reporting that might occur in response to intense media attention and increased public awareness, such as during the 2009 H1N1 pandemic influenza vaccination program. Quality and completeness of VAERS reports are variable and many reports lack valid medical diagnoses. The amount of VAERS reporting (30,000 U.S. reports annually) makes it impractical to conduct detailed follow-up on all reports to obtain missing and incomplete information and correct inconsistencies and errors. Because VAERS data do not include an unvaccinated comparison group, it is not possible to calculate and compare rates of adverse events in vaccinated versus unvaccinated individuals and determine if vaccination is associated with an increased risk of an adverse event […] Except in unambiguous biologically plausible cases (like pain and redness at the injection site), it generally cannot be determined if a vaccine caused an adverse event using VAERS data. On rare occasions, a detailed VAERS report with documentation of conclusive clinical or laboratory evidence might be sufficient to establish causality.” (Page 8)
Shimabukuro, T. T., Nguyen, M., Martin, D., & DeStefano, F. (2015). Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine, 33(36), 4398-4405. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4632204/pdf/nihms-732970.pdf#page=8
- (Suzuki & Gychka, 2021):
Suzuki, Y. J., & Gychka, S. G. (2021). SARS-CoV-2 spike protein elicits cell signaling in human host cells: implications for possible consequences of COVID-19 vaccines. Vaccines, 9(1), 36. DOI: 10.3390/vaccines9010036. https://doi.org/10.3390/vaccines9010036
- (Tuvali et al., 2022):
Tuvali, O., Tshori, S., Derazne, E., Hannuna, R. R., Afek, A., Haberman, D., … & George, J. (2022). The Incidence of Myocarditis and Pericarditis in Post COVID-19 Unvaccinated Patients—A Large Population-Based Study. Journal of Clinical Medicine, 11(8), 2219. DOI: 10.3390/jcm11082219. https://doi.org/10.3390/jcm11082219
- (U.S. CDC, 2022):
Display as Percent because the numbers are only a sampling of 10% of the population; for details, see https://gis.cdc.gov/grasp/COVIDNet/Documents/320393-B_COVID-NET_HospRates-qeo2.pdf
U.S. CDC (2022b). Laboratory-confirmed COVID-19-associated hospitalizations. Retrieved July, 2022, from https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html
- (U.S. CDC, 2022c):
“Anaphylaxis, an acute and potentially life-threatening allergic reaction, has been reported rarely following COVID-19 vaccination. […] symptoms of anaphylaxis might be more difficult to recognize in people with communication difficulties.”
U.S. CDC (2022c). Laboratory-confirmed COVID-19-associated hospitalizations. Retrieved July, 2022, from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html
- (U.S. CDC, 2022d):
U.S. CDC (2022d). New admissions of patients with confirmed COVID-19, United States. Retrieved July, 2022, from https://covid.cdc.gov/covid-data-tracker/#new-hospital-admissions
- (U.S. CDC, 2022e):
“80.1% fully or probably fully recovered” (Page 13)
U.S. CDC (2022e). Update on myocarditis following mRNA COVID-19 vaccination. Retrieved July, 2022, from https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-COVID-Shimabukuro-508.pdf#page=13
- (U.S. CDC, 2022f):
“Rare cases of Bell’s palsy (acute peripheral facial nerve palsy) were reported following vaccination of participants in mRNA COVID-19 vaccine clinical trials, but FDA was not able to determine whether these cases were causally related to vaccination.”
U.S. CDC (2022f). Update on myocarditis following mRNA COVID-19 vaccination. Retrieved July, 2022, from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#special-populations
- (U.S. CDC, 2022g):
“a fifth of all respondents said they would get their child ages 6 months – 4 years vaccinated within 3 months after becoming eligible” (Page 78)
U.S. CDC (2022g). Evidence to Recommendation Framework: Moderna COVID-19 vaccine in children ages 6 months – 5 years & Pfizer-BioNTech COVID-19 vaccine in children ages 6 months – 4 years. Retrieved July, 2022, from https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-17-18/03-COVID-Oliver-508.pdf#page=78
- (U.S. CDC, 2022h):
“Continued monitoring has identified nine deaths causally associated with J&J/Janssen COVID-19 vaccination.”
U.S. CDC (2022h). Selected adverse events reported after COVID-19 vaccination. Retrieved July, 2022, from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- (U.S. CDC, 2022i):
“Expansion of vaccine recommendations down to children 6 months of age would allow 18.7 million children to receive primary COVID-19 vaccine series” (Page 123)
U.S. CDC (2022i). Evidence to Recommendation Framework: Moderna COVID-19 vaccine in children ages 6 months – 5 years & Pfizer-BioNTech COVID-19 vaccine in children ages 6 months – 4 years. Retrieved July, 2022, from https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-17-18/03-COVID-Oliver-508.pdf#page=123
- (U.S. CDC, 2022j):
“Group results by: Year; Ten-Year Age Groups: < 1 year; Select underlying cause of death: U07.1 (COVID-19)
2020 = 35; Crude Rate Per 100,000 (population 3,735,010) = 0.9
2021 (provisional) = 91; Crude Rate Per 100,000 (population 3,735,010) = 2.4
2022 (provisional and partial) = 40; Crude Rate Per 100,000 (population 3,735,010) = 1.1Group results by: Year; Ten-Year Age Groups: 1-4 years; Select underlying cause of death: U07.1 (COVID-19)
2020 = 19; Crude Rate Per 100,000 (population 15,566,282) = 19*100000/15566282 = 0.1
2021 (provisional) = 54; Crude Rate Per 100,000 (population 15,566,282) = 0.3
2022 (provisional and partial) = 31; Crude Rate Per 100,000 (population 15,566,282) = 0.2”U.S. CDC (2022j). Provisional mortality statistics, 2018 through last month request: Deaths occurring through June 25, 2022 as of July 06, 2022. Retrieved July, 2022, from http://wonder.cdc.gov/mcd-icd10-provisional.html
- (U.S. CDC, 2022k):
Influenza virus and pneumonia used as per Flaxman et al. which was used by the CDC committee.
“Group results by: Year; Ten-Year Age Groups: < 1 year; Select underlying cause of death: J019-J18 (Influenza and pneumonia)
2018 = 176; Crude Rate Per 100,000 (population 3,848,208) = 4.6
2019 = 156; Crude Rate Per 100,000 (population 3,783,052) = 4.1
2020 = 125; Crude Rate Per 100,000 (population 3,735,010) = 3.3
2021 (provisional) = 126; Crude Rate Per 100,000 (population 3,735,010) = 3.4
2022 (provisional and partial) = 13; Crude Rate Per 100,000 (population 3,735,010) = 13*100000/3735010 = 0.3Group results by: Year; Ten-Year Age Groups: 1-4 years; Select underlying cause of death: J019-J18 (Influenza and pneumonia)
2018 = 122; Crude Rate Per 100,000 (population 15,962,067) = 0.8
2019 = 122; Crude Rate Per 100,000 (population 15,793,631) = 0.8
2020 = 84; Crude Rate Per 100,000 (population 15,566,282) = 0.5
2021 (provisional) = 48; Crude Rate Per 100,000 (population 15,566,282) = 0.3
2022 (provisional and partial) = 18; Crude Rate Per 100,000 (population 15,566,282) = 18*100000/15566282 = 0.1”U.S. CDC (2022k). Provisional mortality statistics, 2018 through last month request: Deaths occurring through June 25, 2022 as of July 06, 2022. Retrieved July, 2022, from http://wonder.cdc.gov/mcd-icd10-provisional.html
- (U.S. CDC, 2022l):
“Pediatric vaccine preventable diseases: Deaths per year in the United States prior to recommended vaccines” (Page 19)
U.S. CDC (2022l). Evidence to Recommendation Framework: Moderna COVID-19 vaccine in children ages 6 months – 5 years & Pfizer-BioNTech COVID-19 vaccine in children ages 6 months – 4 years. Retrieved July, 2022, from https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-17-18/03-COVID-Oliver-508.pdf#page=19
- (U.S. CDC, 2022m):
U.S. CDC (2022m). Rates of COVID-19 Cases and Deaths by Vaccination Status. Retrieved July, 2022, from https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status
- (U.S. FDA, 2007):
“Mild (Grade 1); Moderate (Grade 2); Severe (Grade 3); Potentially Life Threatening (Grade 4)” (Page 5)
U.S. FDA (2007). Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Retrieved July, 2022, from https://www.fda.gov/media/73679/download#page=5
- (U.S. FDA, 2022):
U.S. FDA (2022). Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. Retrieved July, 2022, from https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children
- (U.S. FDA, 2022b):
“Of children 6 months to 4 years of age with COVID-19 associated hospitalization, 49% had one or more underlying health conditions. The most common underlying medical conditions among hospitalized children (≤18 years) were obesity (31.9%), neurologic disorders (14.8%), and asthma (14.5%). Obesity was associated with increased risk of severe disease. Available evidence suggests that highest risk groups include children with special healthcare needs, including genetic, neurologic, metabolic conditions, or with congenital heart disease. However, a majority of children hospitalized for COVID-19 have no underlying medical conditions.” (Page 9)
U.S. FDA (2022b). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=9
- (U.S. FDA, 2022c):
“randomized, double-blinded, placebo-controlled trial […] 3 μg mRNA per dose or saline placebo” (Page 6)
U.S. FDA (2022c). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=6
- (U.S. FDA, 2022d):
“Table 19. First COVID-19 Occurrence Any Time After Dose 1, Blinded Follow-Up Period, Participants 6-23 Months of Age, All-Available Efficacy Population, Study C4591007 […] ≥7 Days after Dose 3 […] 1 [of] 277 […] 2 [of] 139 […] 75.5” (Page 40)
U.S. FDA (2022d). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=40
- (U.S. FDA, 2022e):
“Table 20. First COVID-19 Occurrence Any Time After Dose 1, Participants 2 to <5 Years of Age, All-Available Efficacy Population, Study C4591007 […] ≥7 Days after Dose 3 […] 2 [of] 481 […] 5 [of] 209 […] 82.3” (Page 40)
U.S. FDA (2022e). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=40
- (U.S. FDA, 2022f):
“Phase 2/3 Overview of Adverse Events” (Page 42)
U.S. FDA (2022f). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=42
- (U.S. FDA, 2022g):
“4.2.7.5. Serious Adverse Events” (Page 52)
U.S. FDA (2022g). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=52
- (U.S. FDA, 2022h):
“4.2.7.7. Adverse events leading to study withdrawal” (Page 56)
U.S. FDA (2022h). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=56
- (U.S. FDA, 2022i):
“From the combined safety database of 3,013 BNT162b2 recipients 6 months through 4 years of age, 1% of participants (n=29) reported SAEs, as compared to 1.5% of participants (n=22) in the combined safety database of 1,513 placebo recipients 6 months through 4 years of age.” (Page 57)
U.S. FDA (2022i). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=57
- (U.S. FDA, 2022j):
“Table 31. Projected COVID-19 Cases, Hospitalizations, and Deaths Averted per Million Over 6 Months With Three Doses of BNT162b2 3 μg in Children 6 Months to <5 Years of Age […] In conclusion, under the pandemic average incidence scenario with assumptions of average 20% (FDA’s estimate), 70% (Sponsor’s lower bound) and 80% (Sponsor’s estimate) VE against COVID-19 cases, COVID-19 hospitalization, and death due to COVID-19, the estimate of vaccine-prevented cases, hospitalizations, and deaths are 5,880, 364, and 5, respectively. The estimate of excess cases of myocarditis/pericarditis per million is 2.5 to 74.0 for males and 0.6 to 7.0 for females, respectively (Sponsor’s estimate), in those 6 months through 4 years of age. FDA reviewers consider the benefits of a three-dose primary series of Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for this age group.” (Pages 62, 63)
U.S. FDA (2022j). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=62
- (U.S. FDA, 2022k):
“The median duration of combined blinded and unblinded follow-up after Dose 3 was 2.1 months for each age group.” (Page 25)
U.S. FDA (2022k). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=25
- (U.S. FDA, 2022l):
“Cumulative incidence curves” (Pages 41, 42)
U.S. FDA (2022l). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=41
- (U.S. FDA, 2022m):
“Participants 6-23 months of age […] No Grade 4 local or systemic reactions were reported after any dose. […] Participants 2-4 years of age […] No Grade 4 local or systemic reactions were reported after any dose.” (Pages 44-46)
U.S. FDA (2022m). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=44
- (U.S. FDA, 2022n):
“There were no reports of myocarditis/pericarditis, no cases of anaphylaxis considered caused by vaccination, and no deaths.” (Page 8)
U.S. FDA (2022n). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=8
- (U.S. FDA, 2022o):
“Vaccine efficacy post-Dose 3 cannot be precisely estimated due to the limited number of cases accrued during blinded follow-up, as reflected in the wide confidence intervals associated with the estimates.” (Page 38)
U.S. FDA (2022o). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=38
- (U.S. FDA, 2022p):
“These descriptive efficacy data are preliminary, as the protocol specified 21 cases have not yet been achieved.” (Page 38)
U.S. FDA (2022p). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=38
- (U.S. FDA, 2022q):
“From Dose 1 through the data cutoff, 1 placebo recipient 6-23 months of age and 7 participants 2-4 years of age (6 BNT162b2 recipients and 1 placebo recipient) met the criteria for severe COVID-19, with only one hospitalization for severe COVID-19 disease in a BNT162b2 recipient 99 days post-Dose 2.” (Page 57)
U.S. FDA (2022q). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=57
- (U.S. FDA, 2022r):
“the primary basis for inference of effectiveness for the 3-dose primary series in individuals 6 months through 4 years of age is based on immunobridging to neutralizing antibody responses among individuals 16-25 years of age who received a 2-dose primary series.” (Page 61)
U.S. FDA (2022r). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=61
- (U.S. FDA, 2022s):
“FDA assessed that the Sponsor may be overestimating VE against COVID-19 cases.” (Page 61)
U.S. FDA (2022s). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=61
- (U.S. FDA, 2022t):
“Based on the real-world data, the average VE within 6 months post-Dose 2 for individuals 5 years and older is approximately 20% (~60% at the beginning and quickly waning afterward) during the Omicron period, 51,52 which is much lower than the VE used as the input of the Sponsor’s benefit-risk assessment. However, it is possible that VE after a 3-dose primary series in individuals 6 months through 4 years of age will be higher than VE after a 2-dose primary series in older age groups.” (Page 61)
U.S. FDA (2022t). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=61
- (U.S. FDA, 2022u):
“A nationwide survey of commercial lab data showed that approximately 75% of unvaccinated individuals among those 0-17 years of age tested positive for antibodies against COVID-19, and some studies suggest that the added protection from vaccination is more limited for those with prior infection within 6 months.” (Page 61)
U.S. FDA (2022u). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=63
- (U.S. FDA, 2022v):
“COVID-19 vaccines represent a new class of vaccines, with many of the lead candidates based on new platform technologies” (Page 16)
U.S. FDA (2022v). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=16
- (U.S. FDA, 2022w):
“The descriptive analyses indicate that post-Dose 3 in both age groups, neutralizing antibody GMTs against the reference strain and against the Delta variant were similar, while neutralizing antibody GMTs against the Omicron variant were notably lower” (Page 36)
U.S. FDA (2022w). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=36
- (U.S. FDA, 2022x):
“Among all COVID-19 cases accrued from Dose 1 through the data cutoff of April 29, 2022, 1 placebo recipient 6-23 months of age and 7 participants 2-4 years of age (6 BNT162b2 recipients and 1 placebo recipient) met the protocol-specified criteria for severe COVID-19 during both blinded and open-label follow-up.” (Page 7)
U.S. FDA (2022x). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=7
- (U.S. FDA, 2022y):
“Myocarditis/pericarditis, in particular in the first week following Dose 2, is a known risk associated with the Pfizer-BioNTech COVID-19 Vaccine and is greatest among adolescent males 16-17 years of age compared with both younger and older age groups.” (Page 65)
U.S. FDA (2022y). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=65
- (U.S. FDA, 2022z):
“Anaphylaxis, primarily among individuals with a history of severe allergic reactions to other medications or foods, has been documented to occur at a rate of approximately 6 cases per million doses among BNT162b2 recipients 16 years of age and older (similar in magnitude to reported rates of anaphylaxis following other licensed preventive vaccines).” (Page 65)
U.S. FDA (2022z). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=65
- (U.S. FDA, 2022aa):
“Available data also do not indicate high-level or durable effectiveness against transmission of SARS-CoV-2 from vaccinated individuals with breakthrough infections.” (Pages 64, 65)
U.S. FDA (2022aa). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=64
- (U.S. FDA, 2022ab):
“based on experience with adults, it is likely that a booster dose will be needed in addition to the three-dose primary series to increase robustness, breadth, and duration of protection against currently circulating and emerging SARS-CoV-2 variants in children 6 months through 4 years of age” (Page 64)
U.S. FDA (2022ab). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=64
- (U.S. FDA, 2022ac):
“In study C4591007, vaccine effectiveness was inferred by immunobridging based on SARS-CoV-2 50% neutralizing antibody titers (NT50, SARS-CoV-2 mNG microneutralization assay). In each of the pre-specified age groups (6-23 months and 2-4 years), neutralizing antibody titers at 1 month post-Dose 3 were compared to titers at 1 month post-Dose 2 from a randomly selected subset of participants 16-25 years of age who had received two doses of 30 μg BNT162b2 in the Phase 2/3 efficacy study, C4591001.” (Page 6)
U.S. FDA (2022ac). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=6
- (U.S. FDA, 2022ad):
“Table 19. First COVID-19 Occurrence Any Time After Dose 1, Blinded Follow-Up Period, Participants 6-23 Months of Age, All-Available Efficacy Population, Study C4591007 […] Dose 1 to before Dose 2 […] -29.7” (Page 40)
U.S. FDA (2022ad). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=40
- (U.S. FDA, 2022ae):
“Table 20. First COVID-19 Occurrence Any Time After Dose 1, Participants 2 to <5 Years of Age, All-Available Efficacy Population, Study C4591007 […] Dose 1 to before Dose 2 […] -32.1” (Page 40)
U.S. FDA (2022ae). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=40
- (U.S. FDA, 2022af):
“Participants enrolled after implementation of protocol amendment 6 will be unblinded at their 6-month post-Dose 3 visit, and those originally randomized to placebo will be offered BNT162b2 vaccination.” (Page 17)
U.S. FDA (2022af). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=17
- (U.S. FDA, 2022ag):
“The uncertainties associated with risks of the Pfizer-BioNTech COVID-19 vaccine when used in children 6 months through 4 years of age include the following: […] Whether the vaccine is associated with subclinical myocarditis, and if so, whether there are long-term sequelae.” (Page 65)
U.S. FDA (2022ag). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=65
- (U.S. FDA, 2022ah):
“The case definition for a severe COVID-19 case included a confirmed COVID-19 case with at least one of the following: • Clinical signs at rest indicative of severe systemic illness (RR and HR, by age, SpO 2≤93% on room air at sea level, or PaO 2/FiO 2 <300 mm Hg) […]” (Page 74)
U.S. FDA (2022ah). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=74
- (U.S. FDA & Pfizer-BioNTech, 2022):
U.S. FDA & Pfizer-BioNTech (2022). Comirnaty and Pfizer-BioNTech COVID-19 Vaccine. Retrieved July, 2022, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine
- (U.S. FDA & Moderna, 2022):
U.S. FDA, & Moderna (2022). Spikevax and Moderna COVID-19 Vaccine. Retrieved July, 2022, from https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine
- (Wise, 2021):
Wise, J. (2021). Long covid: One in seven children may still have symptoms 15 weeks after infection, data show. BMJ. DOI: 10.1136/bmj.n2157. https://doi.org/10.1136/bmj.n2157
- (Woodruff et al., 2022):
Woodruff, R. C., Campbell, A. P., Taylor, C. A., Chai, S. J., Kawasaki, B., Meek, J., … & COVID-NET surveillance team. (2022). Risk factors for severe COVID-19 in children. Pediatrics, 149(1). DOI: 10.1542/peds.2021-053418. https://doi.org/10.1542/peds.2021-053418
- (Yalcinkaya et al., 2022):
Yalçinkaya, R., Öz, F. N., Polat, M., Uçan, B., Teke, T. A., Kaman, A., … & Tanir, G. (2022). A case of multisystem inflammatory syndrome in a 12-year-old male after COVID-19 mRNA vaccine. The Pediatric Infectious Disease Journal, 41(3), e87. DOI: 10.1097/INF.0000000000003432. https://doi.org/10.1097/INF.0000000000003432
- (Yamamoto, 2022):
Yamamoto, K. (2022). Adverse effects of COVID-19 vaccines and measures to prevent them. Virology Journal, 19(1), 1-3. DOI: 10.1186/s12985-022-01831-0. https://doi.org/10.1186/s12985-022-01831-0 ; Recommended: https://virologyj.biomedcentral.com/track/pdf/10.1186/s12985-022-01831-0.pdf
- (Zimmermann et al., 2021):
Zimmermann, P., Pittet, L. F., & Curtis, N. (2021). How common is long COVID in children and adolescents?. The Pediatric infectious disease journal, 40(12), e482. DOI: 10.1097/INF.0000000000003328. https://doi.org/10.1097/INF.0000000000003328
- (Zimmermann et al., 2022):
Zimmermann, P., Pittet, L. F., & Curtis, N. (2022). Long covid in children and adolescents. BMJ, 376. DOI: 10.1136/bmj.o143. https://doi.org/10.1136/bmj.o143