All Vaccines
Quick Summaries
Oversimplified summaries of key points
- Mainstream view steelman: Vaccines are correlated with dramatic reductions of deaths & morbidity, most people being vaccinated helps protect others through herd immunity, there have been multi-year, randomized controlled scientific trials of vaccines versus saline placebo that have not shown concerning all-cause impacts in the vaccine group, and the burden of proof is on those proposing excessive skepticism of vaccines.
- Skeptical of mainstream view steelman: Claims that vaccines dramatically reduced deaths & morbidity are based on very incomplete models, recent natural experiments of the DTP vaccine in Africa have shown multiple times worse all-cause mortality in the vaccine group, herd immunity doesn’t apply to many vaccines, post-approval surveillance may report fewer than 1% of adverse events, 70% of scientific vaccine trials of currently licensed vaccines in the U.S. were based on studies that did not use a control or did not use saline placebo, vaccines such as the Hepatitis B vaccine given to children on the first day of life in the U.S. were based on studies without any control and babies were monitored for less than 6 days, some saline placebo randomized & controlled trials have shown worse severe disease in the vaccine group after many years particularly in children, and the burden of proof is on those proposing to avoid performing larger, randomized, controlled, saline placebo trials and/or epidemiological investigations of vaccinated versus under- or un-vaccinated.
Background on Vaccines
The immune system attacks perceived threats (called pathogens) through mechanisms such as adaptive defenses that learn to respond to antigens that are molecules on or released by pathogens (Lam et al., 2024).
This learning process includes creating B and T memory cells that may respond more quickly to future re-infections by the same or similar antigens (Lam et al., 2024).
Vaccines are injections, sprays, drops, etc. containing antigens or something that creates antigens with the goal of stimulating such immunological memory (Papania et al., 2017).
Vaccines contain other ingredients including adjuvants that stimulate the immune system (e.g. aluminum, squalene oil, polysorbate, etc.), antibiotics (e.g. mercury) & preservatives that reduce risks of contamination, stabilizers that reduce changes due to environmental exposures, surfactants, diluents, and residual materials from the production process (e.g. virus molecules, bacteria molecules, DNA, cell culture residuals, etc.) (Vanderslott et al., 2019; Children’s Hospital of Philadelphia, 2025; Offit & Jew, 2003; Delany et al., 2014).
A normal part of the immune response involves inflammation and increased blood flow and this may cause fever, redness, swelling, pain, aches, etc. and it’s expected that this inflammation may occur both with pathogens and vaccines (Hervé et al., 2019).
The following steelmen are for general points across all vaccines and separate steelmen are needed for each specific vaccine.
Steelman: Mainstream View
- Epidemiology:
- Modeling shows vaccines avoided over 150 million deaths (including over 100 million infants) over the past 50 years worldwide (Shattock et al., 2024; Zhou et al., 2024; Rodrigues & Plotkin, 2020; Zhou et al., 2005; Zhou et al., 2014; Whitney et al., 2014; Roush et al., 2007; U.S. CDC, 1999b) and annually reduce severe disease, hospitalizations and impact up to over $1 trillion in reduced medical costs and increased economic benefits in the U.S. as an example (Ozawa et al., 2016).
- A natural experiment occurred when concerns about DTwP vaccine side effects led to reduced pertussis vaccination in countries such as Japan that was correlated with increases of dozens of pertussis deaths in the late 1970s; then, when an acellular version of the vaccine (DTaP) was introduced, pertussis cases and deaths subsequently declined in the early 1980s (Kimura & Kuno-Sakai, 1990; Ellenberg & Chen, 1997). A similar thing happened with whooping cough and pertussis cases in the U.S. in the 1970s & 1980s (Rawlins, 1995b).
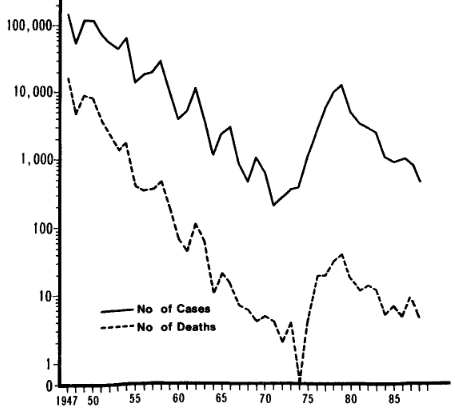
Reported cases of pertussis and deaths (A) (Kimura & Kuno-Sakai, 1990)
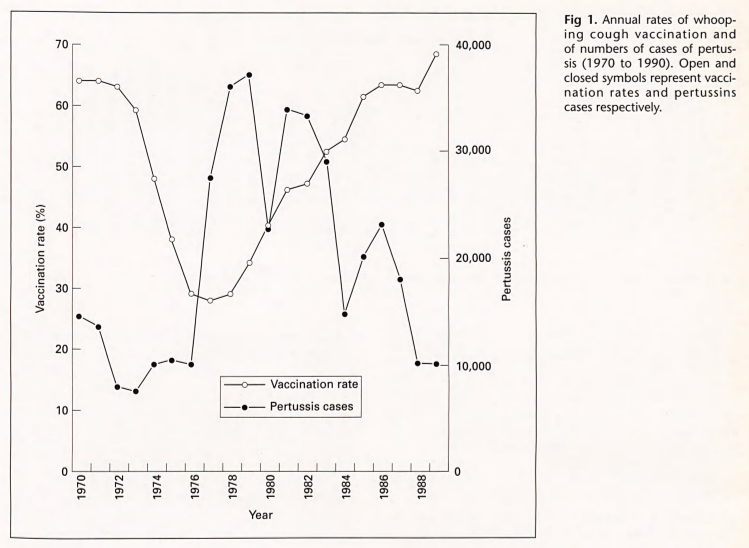
Fig 1. Annual rates of whooping cough vaccination and of numbers of cases of pertussis (1970 to 1990). Open and closed symbols represent vaccination rates and pertussins cases respectively. (Rawlins, 1995b) - Insufficient community vaccination may endanger those that cannot get a vaccine due to being immunocompromised (e.g. cancer treatment, etc.), contraindications, too young, ineffective immune response, cost, availability, etc. because of reduced herd immunity (Rodrigues & Plotkin, 2020d; Orenstein & Ahmed, 2017; Zimmermann & Curtis, 2019).
- Vaccines eliminated smallpox, polio (in advanced countries), and rinderpest (in livestock) thus completely eliminating those pathogens and the morbidity and mortality they cause (Parrino & Graham, 2006; Henderson, 2011; Bandyopadhyay et al., 2015). Smallpox had been around since at least 1,000 BC (Rodrigues & Plotkin, 2020b).
- There are no known causal relationships between vaccines and chronic or autoimmune diseases (e.g. asthma, multiple sclerosis, diabetes, hay fever, etc.) (Offit & Jew, 2003; McCormick et al., 2002).
- Safety:
- About 30% of currently approved vaccines in the U.S. were approved based on randomized controlled trials that used inactive saline placebo with placebo groups up to 18,098 patients (average of 2,181) and monitoring for severe adverse reactions up to 6 years (average of about 10 months) with similar rates of reported severe adverse reactions and all cause mortality (Steelman Anything, 2025; U.S. FDA, 2006; U.S. FDA, 2007; U.S. FDA, 2009d; U.S. FDA, 2009g; U.S. FDA, 2013; U.S. FDA, 2015; U.S. FDA, 2019; U.S. FDA, 2019c; U.S. FDA, 2020; U.S. FDA, 2020b; U.S. FDA, 2023f; U.S. FDA, 2023j; U.S. FDA, 2023k; U.S. FDA, 2023l; U.S. FDA, 2023m; U.S. FDA, 2023s; U.S. FDA, 2024d; U.S. FDA, 2024e; U.S. FDA, 2024f; U.S. FDA, 2024g; U.S. FDA, 2024h; U.S. FDA, 2024n; U.S. FDA, 2024o; U.S. FDA, 2024p; U.S. FDA, 2024q; U.S. FDA, 2024s; U.S. FDA, 2024t; U.S. FDA, 2025; U.S. FDA, 2025f; U.S. FDA, 2025h; U.S. FDA, 2025i; U.S. FDA, 2025l; Rid et al., 2014).
- About half of saline placebo studies included children younger than 18 years old and as young as 6 months with placebo groups up to 1,354 patients (average of 647) and monitoring for severe adverse reactions up to 6 years (average of about 9 months) with similar rates of reported severe adverse reactions and all cause mortality (Steelman Anything, 2025; U.S. FDA, 2006; U.S. FDA, 2009d; U.S. FDA, 2013; U.S. FDA, 2019; U.S. FDA, 2023k; U.S. FDA, 2023m; U.S. FDA, 2023s; U.S. FDA, 2024e; U.S. FDA, 2024p; U.S. FDA, 2024q; U.S. FDA, 2024s; U.S. FDA, 2025; U.S. FDA, 2025i; U.S. FDA, 2025l).
- For example, a randomized controlled trial using inactive saline placebo for the Zostavax shingles vaccine trial in adults included thousands of placebo recipients monitored for about 5 years and did not show statistically significantly more reported severe disease, hospitalization or all cause mortality (U.S. FDA, 2019c).
- Vaccinations correlated with serious adverse events such as sudden infant death syndrome (SIDS), etc. may be spurious correlations (Ellenberg & Braun, 2002).
- Post-approval surveillance is used to monitor for side effects correlated with vaccines (Di Pasquale et al., 2016; U.S. CDC, 2024b) and studied extensively (U.S. CDC, 2024). Documented serious adverse events are extremely rare (Maglione et al., 2014b) though there are various limitations to surveillance systems such as the U.S. VAERS and VSD systems (Salmon et al., 2024b) and some rare serious adverse events are unavoidable (Chen et al., 2001). In the U.S., a vaccine injury court exists though some rulings have a low barrier of plausibility (Offit, 2008).
- There’s no strong evidence that there are problems receiving multiple vaccines at the same time (including as an infant) or when someone is non-severely ill (Offit et al., 2002).
- Given that vaccines are correlated with dramatically reduced deaths, morbidity, and hospitalizations, that vaccine skepticism has been correlated with spikes of deaths and morbidity, that most individuals being vaccinated may protect vulnerable people that can’t take vaccines or for whom vaccines are ineffective through herd immunity, that vaccines are correlated with full or near elimination of smallpox and polio, that randomized & controlled vaccine scientific trials show safety and efficacy even over multi-year, saline placebo randomized & controlled clinical trials, that potential adverse effects continue to be investigated and rare adverse events compensated, and that it’s widely believed to be unethical and impractical to perform better randomized & controlled trials, then the burden of proof is on those proposing excessive skepticism of vaccines.
Steelman Response: Skeptical of Mainstream View
- Epidemiology:
- The modeling of deaths, hospitalizations and costs saved by vaccines may be a spurious correlation because at least 96.5% to 99.0% of the reduction in infectious disease mortality in advanced countries occurred before vaccines were widely available (McKinlay & McKinlay, 1977; Guyer et al., 2000; Kass, 1971; McKeown & Brown, 1955; Cutler & Miller, 2005), paralelled by non-vaccine treated diseases such as typhoid, scarlet fever, etc. (Vanderslott et al., 2019; Kass, 1971). Recent reductions of disease impacts in poorer countries may have had similar drivers (Mahmood et al., 2014; Cutler et al., 2006). This is in addition to other potential epidemiological confounders such as healthy vaccinee bias (Remschmidt et al., 2015).
- For example, in the United States, measles vaccines were introduced in 1963 (Conis, 2019) yet most of the reduction in measles deaths occurred before (first figure below) (Grove & Hetzel, 1968; Kass, 1971). Similarly, diphtheria vaccine was introduced in 1913 (Colgrove, 2007) yet didn’t accelerate the rate of diphtheria death decline (second figure below) (Grove & Hetzel, 1968b; Kass, 1971). As a third example, a vaccine for pertussis came well after whooping cough deaths declined dramatically (third figure below) (Kass, 1971). There are similar trends for polio, influenza, pneumonia, tuberculosis, etc. (Kass, 1971; McKinlay & McKinlay, 1977; Guyer et al., 2000). Modeling also cannot take into account what may have continued to happen to trends without vaccines and all-cause effects (i.e. those still affected may be equally or worse affected by other ailments).
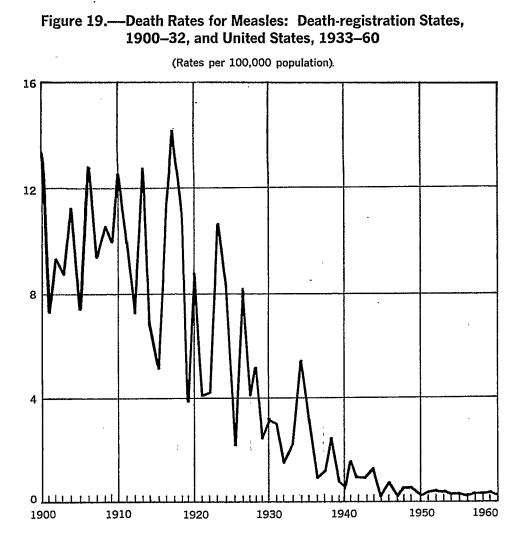
Figure 19.—Death Rates for Measles: Death-registration States, 1900-32, and United States, 1933-60; (Rates per 100,000 population). (Grove & Hetzel, 1968)
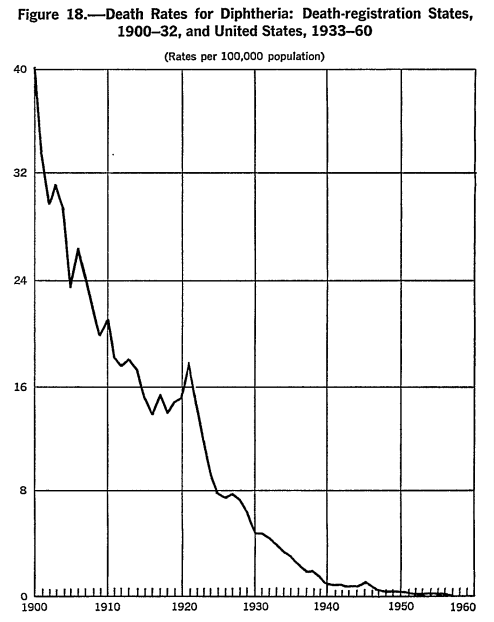
Figure 18.—Death Rates for Diphtheria: Death-registration States, 1900-32, and United States, 1933-60; (Rates per 100,000 population). (Grove & Hetzel, 1968b)

Figure 4. Mean annual death rate from whooping cough in children under 15 years of age, England and Wales (Kass, 1971) - A natural, uncontrolled experiment of the introduction of the DTP vaccine (one of the most widely used vaccines globally) in Africa in the 1970s and 1980s showed at least two fold, statistically significant worse all-cause mortality in the vaccine group which was hypothesized to be due to non-specific effects (NSEs) of non-live attenuated vaccines (Aaby et al., 2018; Andersen et al., 2018; Mogensen et al., 2017; Aaby et al., 2016; Higgins et al., 2016; Aaby et al., 2003; Benn, 2025; Benn et al., 2023; Benn et al., 2020; Bardenheier et al., 2017).
- Some correlational evidence shows that all-cause mortality isn’t statistically significantly different between undervaccinated or unvaccinated children compared to children vaccinated per the recommended U.S. schedule (McCarthy et al., 2017).
- The number of vaccines and/or antigens or other ingredients in vaccines given in combination or in total may have increased risks (Institute of Medicine, 2013b; Ellenberg & Chen, 1997d; Goldman, 2013; Von Kries et al., 2005; Traversa et al., 2011; Australia Government, 2007; Jablonowski & Hooker, 2024). Some evidence shows the number of vaccines given is correlated with increased infant mortality (Miller & Goldman, 2011; Miller & Goldman, 2023; Goldman & Miller, 2023; Jablonowski & Hooker, 2025). Infant deaths (e.g. SIDS) reported to the U.S. Vaccine Adverse Event Reporting System (VAERS) system showed that the number of days since vaccination was not randomly distributed (Miller, 2021).
- Herd immunity is particularly effective when the pathogen is casually contagious and the vaccine reduces transmission. Tetanus is not contagious (Hall et al., 2021c). Diphtheria vaccines may not significantly prevent transmission (Miller et al., 1972). Acellular pertussis vaccines may not significantly prevent transmission (Warfel et al., 2014; Warfel & Merkel, 2014). Influenza vaccines may not significantly prevent transmission (Jefferson et al., 2010; Ohmit et al., 2013). The MMR (Measles, Mumps and Rubella) vaccine may wane significantly over time and that is not significantly helped with a third booster (Fiebelkorn et al., 2016). The COVID-19 vaccine may not significantly prevent transmission (Riemersma et al., 2022). The inactivated polio vaccine (IPV) may not significantly prevent transmission (U.S. CDC, 2024c). Hepatitis B is mainly spread through sexual contact, injection drug use, or being born to an infected mother (Hall et al., 2021). HPV is a sexually transmitted disease (Hall et al., 2021b).
- Safety:
- Despite the lack of evidence of a significant difference in severe adverse reactions and all cause mortality in saline placebo randomized controlled vaccine trials, their sample sizes are primarily designed to evaluate efficacy rather than the safety, including in subpopulations, and may purposefully exclude those who may be more susceptible (Maglione et al., 2014; Chen, 1999c; Ellenberg et al., 2005b; Ellenberg & Braun, 2002b). Due to the nature of the statistics used, studies may also lack the ability to detect adverse effects that are otherwise common in the population (Brewer & Colditz, 1999).
- Vaccines are accepted by mainstream science to cause at least a small number of serious adverse outcomes (Ellenberg et al., 2005; Poland & Kennedy, 2022) including death (U.S. CDC, 2022); although these should be evaluated relative to the risks of the vaccine-precentable disease, post-approval surveillance found correlations with intussusception (U.S. CDC, 1999), thrombocytopenia (U.S. CDC, 2022; World Health Organization, 2023; Ellenberg & Chen, 1997b), narcolepsy (Nohynek et al., 2012; Persson et al., 2014), myopericarditis and ischemic cardiac events (Varricchio et al., 2004b; Le Vu et al., 2022; U.S. FDA, 2022y; Chua et al., 2021; Buchan et al., 2022), Guillain-Barré syndrome (Schonberger et al., 1979), vaccine-induced polio and paralysis (Rodrigues & Plotkin, 2020c; Offit, 2005; Alexander et al., 2004), encephalopathy (Chen, 1999), neurological illness (Miller et al., 1981), aseptic meningitis (DeStefano et al., 2001), anaphylactic shock (Ellenberg et al., 2005), viscerotropic and neurotropic disease (Varricchio et al., 2004), seizures (Ellenberg & Chen, 1997c), and muscle pain, abdominal pain, peripheral edema, and pneumonia (Fulginiti et al., 1967; Rauh & Schmidt, 1965). In addition, various manufacturing and handling errors have caused serious illness and death (Baker, 2008b; Offit, 2005; Amanna & Slifka, 2018; Zhou et al., 2003b; Wilson, 1967; U.S. CDC, 2022) and exposure to other viruses such as SV-40 (Ellenberg et al., 2005c). Positive rechallenging has also suggested additional adverse events such as hair loss for some vaccines (Ellenberg & Braun, 2002d).
- There are case reports of identical twins both dying shortly after vaccination (Balci et al., 2007; Mitchell et al., 2010; Roberts, 1987; Werne & Garrow, 1946).
- The U.S. National Vaccine Injury Compensation Program has awarded $5.4 billion to 12,339 petitions since 1988, at least 3,626 of which were conceeded to be more likely than not that the vaccine caused the injury or the evidence supported fulfillment of the criteria of the Vaccine Injury Table (U.S. Health Resources & Services Administration, 2025; U.S. Health Resources & Services Administration, 2025b).
- Even if it’s true that vaccine side affects are rare, vaccines are generally given preventively which may change ethical considerations and the burden of proof of safety studies, especially for healthy infants (Chen, 1999b), and adverse events correlated with vaccines now exceed the incidences of most vaccine-preventable childhood diseases (Chen, 1999d).
- Post-approval surveillance may report fewer than 1% of vaccine adverse events (Lazarus et al., 2010; U.S. Committee on Government Reform, 2000), potential long-term adverse events are less likely to be reported (Ellenberg & Braun, 2002c; Hasford et al., 2002; Rawlins, 1988; Feely et al., 1990), about 14% are for serious adverse events (Zhou et al., 2003b), the system has major weaknesses (Block, 2023; Zhou et al., 2003), and major vaccine safety questions aren’t well studied (Salmon et al., 2024).
- There are statistically significant correlations between vaccination and chronic health conditions such as asthma, atopic disease, eczema, autoimmune disease and neurodevelopmental disorders when comparing vaccinated versus unvaccinated children (Lamerato et al., 2020; Cowling et al., 2012; Institute of Medicine, 2013; Glanz et al., 2016; Mawson et al., 2017; Hooker & Miller, 2020; Hooker & Miller, 2021; Lyons-Weiler & Thomas, 2020; Enriquez et al., 2005).
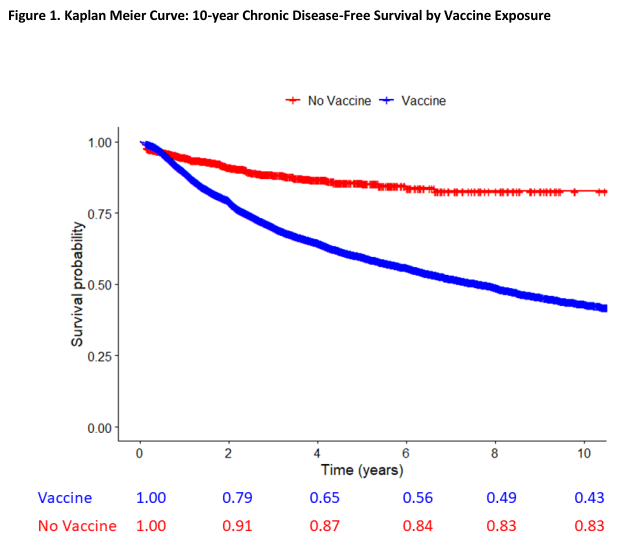
Figure 1. Kaplan Meier Curve: 10-year Chronic Disease-Free Survival by Vaccine Exposure (Lamerato et al., 2020) - About 70% of currently approved vaccines in the U.S. were approved based on safety studies that either were not randomized with a control arm (Steelman Anything, 2025; U.S. FDA, 1978; U.S. FDA, 2009f; U.S. FDA, 2017; U.S. FDA, 2017d; U.S. FDA, 2018; U.S. FDA, 2018d; U.S. FDA, 2018f; U.S. FDA, 2019b; U.S. FDA, 2022; U.S. FDA, 2022e; U.S. FDA, 2023u; U.S. FDA, 2024l; U.S. FDA, 2024m) or the randomized controls were active controls such as other vaccines, adjuvants, preservatives, etc. (Steelman Anything, 2025; Tramer et al., 1998; U.S. FDA, 1998; U.S. FDA, 2009; U.S. FDA, 2009b; U.S. FDA, 2009c; U.S. FDA, 2009e; U.S. FDA, 2018c; U.S. FDA, 2018e; U.S. FDA, 2020c; U.S. FDA, 2020d; U.S. FDA, 2021; U.S. FDA, 2021b; U.S. FDA, 2022c; U.S. FDA, 2022h; U.S. FDA, 2022i; U.S. FDA, 2022j; U.S. FDA, 2022k; U.S. FDA, 2022d; U.S. FDA, 2022b; U.S. FDA, 2023; U.S. FDA, 2023b; U.S. FDA, 2023c; U.S. FDA, 2023d; U.S. FDA, 2023e; U.S. FDA, 2023g; U.S. FDA, 2023r; U.S. FDA, 2023h; U.S. FDA, 2023i; U.S. FDA, 2023o; U.S. FDA, 2023q; U.S. FDA, 2023t; U.S. FDA, 2023w; U.S. FDA, 2023x; U.S. FDA, 2023y; U.S. FDA, 2023z; U.S. FDA, 2024; U.S. FDA, 2024b; U.S. FDA, 2024c; U.S. FDA, 2024i; U.S. FDA, 2024j; U.S. FDA, 2024k; U.S. FDA, 2024r; U.S. FDA, 2024u; U.S. FDA, 2024v; U.S. FDA, 2024w; U.S. FDA, 2024x; U.S. FDA, 2024y; U.S. FDA, 2025b; U.S. FDA, 2025c; U.S. FDA, 2025d; U.S. FDA, 2025e; U.S. FDA, 2025g; U.S. FDA, 2025j). In some randomized controlled trials with inactive placebo, results were statistically significantly worse in the vaccine group (Liang et al., 2010; Hoberman et al., 2003). Active vaccine controls are sometimes used due to ethical concerns (Rid et al., 2014b); however, active controls are generally only allowed when they were themselves tested against inactive placebo (U.S. FDA, 2016). In some cases, the active controls were themselves never tested against saline placebo such as Prevnar 13 being tested against Prevnar 7 but Prevnar 7 not being tested against saline placebo (nor its meningococcal group C conjugate active control) and the Prevnar 13 trials showed up to 5.8% rates of serious adverse events including up to 0.29% rates of death (U.S. FDA, 2002; U.S. FDA, 2017). Three groups could be used to evaluate inactive placebo versus active control versus vaccine (U.S. FDA, 2001).
- For example, all hepatitis B vaccines given to babies on the day of birth in the U.S. were trialed without any control (neither saline nor active control) and babies were monitored for less than 6 days (U.S. FDA, 2018b; U.S. FDA, 2023v; U.S. FDA, 1998b).
- Most routine childhood vaccines in the U.S. were licensed without inactive saline placebo trials (U.S. FDA, 1978; U.S. FDA, 1998; U.S. FDA, 2017; U.S. FDA, 2018; U.S. FDA, 2018c; U.S. FDA, 2020c; U.S. FDA, 2022; U.S. FDA, 2022c; U.S. FDA, 2022e; U.S. FDA, 2022k; U.S. FDA, 2023; U.S. FDA, 2023b; U.S. FDA, 2023o; U.S. FDA, 2023t; U.S. FDA, 2023u; U.S. FDA, 2023w; U.S. FDA, 2023x; U.S. FDA, 2023y; U.S. FDA, 2023z; U.S. FDA, 2024w; U.S. FDA, 2024y; U.S. FDA, 2025j).
- As one example of the possible importance of long trial durations, a randomized controlled trial using inactive saline placebo for the Dengvaxia dengue vaccine with thousands of participants and monitoring for 6 years showed initially positive results but after a few years showed statistically significantly more reported severe disease and hospitalization in the vaccinated, particularly the young, probably due to antibody-dependent enhancement (ADE) (Mersha et al., 2024) (though all-cause mortality was similar) and thus the vaccine is generally not recommended for young or previously uninfected persons in non-endemic areas (U.S. FDA, 2023n; Hadinegoro et al, 2015). This supports the idea of long, large, inactive placebo controlled trials, especially for children. ADE has been seen in at least influenza viruses, flaviviruses, coronaviruses, ebolaviruses, HIV, RSV, measles virus, and dengue virus (Mersha et al., 2024b).
- There may be potential allergic reactions to egg proteins, yeast proteins, gelatin, etc. (Offit & Jew, 2003).
- Even if it’s true that vaccines are correlated with reductions in the incidences of diseases as in the Japanese pertussis example or with the recent introduction of vaccines in poorer countries (Rodrigues & Plotkin, 2020e), other aspects would need to be evaluated such as economic drivers that lead to better nutrition, sanitation, etc. (Sala-i-Martin, 2006) and all-cause mortality & morbidity (e.g. those that died due to that reduction in Japanese pertussis vaccination may have been frail and died due to other causes), net risks & benefits, etc.
- Given that modeling of vaccine impacts on reduced deaths, morbidity, and hospitalizations mostly ignores the impacts of other drivers of reductions such as when nearly all of a disease reduction happened before a vaccine was even introduced or when the introduction of a vaccine didn’t accelerate disease reduction, that spikes of deaths and morbidity correlated with vaccine skepticism hasn’t taken into account all-cause deaths and morbidity, that recent natural experiments of the DTP vaccine in Africa have shown statistically significant, multiple times worse all-cause mortality in the vaccine group, that some correlational evidence shows all-cause mortality isn’t significantly different between vaccinated and undervaccinated or fully unvaccinated children, that some correlational evidence shows the number of vaccines given is correlated with increased infant mortality, that herd immunity doesn’t apply to many vaccines, that randomized & controlled vaccine scientific trials are not robustly designed to evaluate safety of adverse events, that some serious vaccine adverse effects & deaths are documented and accepted even by mainstream science, that billions of dollars of compensation have been paid in the U.S. for vaccine injuries, that adverse events of vaccines generally exceed the incidences of most childhood diseases in advanced countries, that post-approval surveillance may report fewer than 1% of adverse events, that there are significant correlations between vaccination and chronic health conditions, that 70% of scientific vaccine trials of currently licensed vaccines in the U.S. were based on studies that did not use a control or did not use saline placebo and often the vaccine used as controls weren’t themselves not studied versus saline placebo despite some cases of high adverse event rates and death, that vaccines such as the Hepatitis B vaccine given to children on the first day of life in the U.S. were based on studies without any control and babies were monitored for less than 6 days, that most routine childhood vaccines in the U.S. were licensed without inactive saline placebo trials, and that some saline placebo randomized & controlled trials have shown worse severe disease in the vaccine group after many years particularly in children, then the burden of proof is on those proposing to avoid performing larger, randomized, controlled, saline placebo trials and/or epidemiological investigations of vaccinated versus under- or un-vaccinated.
Notes
- Inactive placebo for muscular injections is normally saline. Saline placebo is preferred over purified water for bloodstream injections because saline placebo is isotonic with blood plasma (Wilcox, 1983). Non-saline water would be hypotonic and could cause extra blood cell death; however, a balanced crystalloid solution might be better than saline (Hammond et al., 2022).
Click here to report problems and/or suggestions for this page (requires a free GitHub.com account).
References
295 references
- (Aaby et al., 1995):
Aaby, P., Samb, B., Simondon, F., Seck, A. M. C., Knudsen, K., & Whittle, H. (1995). Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. Bmj, 311(7003), 481-485. DOI: 10.1136/bmj.311.7003.481. https://doi.org/10.1136/bmj.311.7003.481 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC2550544/pdf/bmj00606-0023.pdf
- (Aaby et al., 2003):
“high-titre measles vaccination is believed to cause increased female mortality, whereas no problem is recorded with medium-titre and standard-titre measles vaccines.”
Aaby, P., Jensen, H., Samb, B., Cisse, B., Sodemann, M., Jakobsen, M., … & Whittle, H. (2003). Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. The Lancet, 361(9376), 2183-2188. DOI: 10.1016/S0140-6736(03)13771-3. https://doi.org/10.1016/S0140-6736(03)13771-3
- (Aaby et al., 2016):
“in these studies DTP was associated with an MRR of 2.00 (1.50–2.67). […] Bias does not seem to explain why DTP is associated with higher mortality.”
Aaby, P., Ravn, H., & Benn, C. S. (2016). The WHO review of the possible nonspecific effects of diphtheria-tetanus-pertussis vaccine. The Pediatric infectious disease journal, 35(11), 1247-1257. DOI: 10.1097/INF.0000000000001269. https://doi.org/10.1097/INF.0000000000001269
- (Aaby et al., 2018):
Aaby, P., Mogensen, S. W., Rodrigues, A., & Benn, C. S. (2018). Evidence of increase in mortality after the introduction of diphtheria–tetanus–pertussis vaccine to children aged 6–35 months in Guinea-Bissau: a time for reflection?. Frontiers in public health, 6, 79. DOI: 10.3389/fpubh.2018.00079. https://doi.org/10.3389/fpubh.2018.00079 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2018.00079/pdf
- (Alexander et al., 2004):
“as a consequence of oral poliovirus vaccine (OPV) use that began in 1961, an average of 9 cases of vaccine-associated paralytic poliomyelitis (VAPP) were confirmed each year from 1961 through 1989. To reduce the VAPP burden, national vaccination policy changed in 1997 from reliance on OPV to options for a sequential schedule of inactivated poliovirus vaccine (IPV) followed by OPV. In 2000, an exclusive IPV schedule was adopted.”
Alexander, L., Seward, J. F., Santibanez, T. A., Pallansch, M. A., Kew, O. M., Prevots, D. R., … & Sutter, R. W. (2004). Vaccine policy changes and epidemiology of poliomyelitis in the United States. Jama, 292(14), 1696-1701. DOI: 10.1001/jama.292.14.1696. https://doi.org/10.1001/jama.292.14.1696
- (Amanna & Slifka, 2018):
“the hospitalization rates among infected children in the FIRSV cohort reached 80% (compared to a rate of only 5% in the control group) and the vaccine was associated with two deaths”
Amanna, I. J., & Slifka, M. K. (2018). Successful vaccines. Vaccination strategies against highly variable pathogens, 1-30. DOI: 10.1007/82_2018_102. https://doi.org/10.1007/82_2018_102
- (Andersen et al., 2018):
Andersen, A., Fisker, A. B., Rodrigues, A., Martins, C., Ravn, H., Lund, N., … & Aaby, P. (2018). National immunization campaigns with oral polio vaccine reduce all-cause mortality: a natural experiment within seven randomized trials. Frontiers in public health, 6, 13. DOI: 10.3389/fpubh.2018.00013. https://doi.org/10.3389/fpubh.2018.00013 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2018.00013/pdf
- (Aronson, 2017):
Aronson, J. K. (2017). Post-marketing drug withdrawals: pharmacovigilance success, regulatory problems. Therapies, 72(5), 555-561. DOI: 10.1016/j.therap.2017.02.005. https://doi.org/10.1016/j.therap.2017.02.005
- (Australia Government, 2007):
” Analysis of postmarketing reporting rates suggests a potential increased risk of convulsions (with or without fever) and HHE when comparing groups which reported use of INFANRIX HEXA with Prevenar 13 to those which reported use of INFANRIX HEXA alone.”
U.S. FDA. (2007). INFANRIX HEXA (Combined Diphtheria-Tetanus-acellular Pertussis (DTPa), Hepatitis B, Poliovirus and Haemophilus influenzae type b vaccine) powder and suspension for suspension for injection. Retrieved November, 2025, from http://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2010-PI-06624-3
- (Baker, 2008):
Baker, J. P. (2008). Mercury, vaccines, and autism: one controversy, three histories. American journal of public health, 98(2), 244-253. DOI: 10.2105/AJPH.2007.113159. https://doi.org/10.2105/AJPH.2007.113159 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC2376879/pdf/0980244.pdf
- (Baker, 2008b):
“One of the most troublesome safety issues afflicting early 20th-century child immunization was that of bacterial contamination. […] In Columbia, South Carolina, in 1916, a tainted batch of typhoid vaccine stored at room temperature caused 68 severe reactions, 26 abscesses, and 4 deaths. A still more disturbing incident took place in 1928 in Queensland, Australia, where 12 of 21 children inoculated with contaminated diphtheria vaccine died of multiple staphylococcal abscesses and toxemia”
Baker, J. P. (2008). Mercury, vaccines, and autism: one controversy, three histories. American journal of public health, 98(2), 244-253. DOI: 10.2105/AJPH.2007.113159. https://doi.org/10.2105/AJPH.2007.113159 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC2376879/pdf/0980244.pdf
- (Balci et al., 2007):
Balci, Y., Tok, M., Kocaturk, B. K., Yenilmez, Ç., & Yorulmaz, C. (2007). Simultaneous sudden infant death syndrome. Journal of Forensic and Legal Medicine, 14(2), 87-91. DOI: 10.1016/j.jcfm.2006.01.004. https://doi.org/10.1016/j.jcfm.2006.01.004
- (Bandyopadhyay et al., 2015):
Bandyopadhyay, A. S., Garon, J., Seib, K., & Orenstein, W. A. (2015). Polio vaccination: past, present and future. Future microbiology, 10(5), 791-808. DOI: 10.2217/fmb.15.19. https://doi.org/10.2217/fmb.15.19 ; Recommended: https://www.tandfonline.com/doi/pdf/10.2217/fmb.15.19
- (Bardenheier et al., 2017):
Bardenheier, B. H., McNeil, M. M., Wodi, A. P., McNicholl, J. M., & DeStefano, F. (2017). Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005–2014. Clinical Infectious Diseases, 65(5), 729-737. DOI: 10.1093/cid/cix442. https://doi.org/10.1093/cid/cix442 ; Recommended: https://academic.oup.com/cid/article-pdf/65/5/729/19832657/cix442.pdf
- (Benn, 2025):
“There is now compelling evidence that not only do vaccines have specific effects against the targeted infections, but they also have non-specific effects (NSEs) on the immune system’s ability to combat other pathogens.”
Benn, C. S. (2025). Non-specific effects of vaccines: The status and the future. Vaccine, 51, 126884. DOI: 10.1016/j.vaccine.2025.126884. https://doi.org/10.1016/j.vaccine.2025.126884
- (Benn et al., 2020):
Benn, C. S., Fisker, A. B., Rieckmann, A., Sørup, S., & Aaby, P. (2020). Vaccinology: time to change the paradigm?. The lancet infectious diseases, 20(10), e274-e283. DOI: 10.1016/S1473-3099(19)30742-X. https://doi.org/10.1016/S1473-3099(19)30742-X
- (Benn et al., 2023):
Benn, C. S., Amenyogbe, N., Björkman, A., Domínguez-Andrés, J., Fish, E. N., Flanagan, K. L., … & Aaby, P. (2023). Implications of non-specific effects for testing, approving, and regulating vaccines. Drug Safety, 46(5), 439-448. DOI: 10.1007/s40264-023-01295-3. https://doi.org/10.1007/s40264-023-01295-3 ; Recommended: https://link.springer.com/content/pdf/10.1007/s40264-023-01295-3.pdf
- (Block, 2023):
Block, J. (2023). Is the US’s vaccine adverse event reporting system broken?. bmj, 383. DOI: 10.1136/bmj.p2582. https://doi.org/10.1136/bmj.p2582 ; Recommended: https://www.bmj.com/content/383/bmj.p2582.full.pdf
- (Brewer & Colditz, 1999):
“Despite important progress in evaluating ADRs [adverse drug reactions], there still is no reliable method for identifying potential ADRs that occur widely separated in time from the original use of a drug, occur with measurable frequency in the unexposed population, and have no predictable relationship to the major effects of the drug. These ADRs are not reliably detected with spontaneous reporting systems”
Brewer, T., & Colditz, G. A. (1999). Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. Jama, 281(9), 824-829. DOI: 10.1001/jama.281.9.824. https://doi.org/10.1001/jama.281.9.824
- (Brisson et al., 2002):
“Mass varicella vaccination is expected to cause a major epidemic of herpes-zoster, affecting more than 50% of those aged 10–44 years at the introduction of vaccination.”
Brisson, M., Gay, N. J., Edmunds, W. J., & Andrews, N. J. (2002). Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine, 20(19-20), 2500-2507. DOI: 10.1016/S0264-410X(02)00180-9. https://doi.org/10.1016/S0264-410X(02)00180-9
- (Buchan et al., 2022):
Buchan, S. A., Seo, C. Y., Johnson, C., Alley, S., Kwong, J. C., Nasreen, S., … & Wilson, S. E. (2022). Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Network Open, 5(6), e2218505-e2218505. DOI: 10.1001/jamanetworkopen.2022.18505. https://doi.org/10.1001/jamanetworkopen.2022.18505
- (Chen, 1999):
“In 1971, the US stopped routine smallpox vaccinations prior to global smallpox eradication due to the burden of vaccine-associated encephalopathy.”
Chen, R. T. (1999). Vaccine risks: real, perceived and unknown. Vaccine, 17, S41-S46. DOI: 10.1016/S0264-410X(99)00292-3. https://doi.org/10.1016/S0264-410X(99)00292-3
- (Chen, 1999b):
“In contrast to most pharmaceutical products, which are administered to ill persons for curative purposes, vaccines are generally given to healthy persons to prevent disease. As an extension of the medical maxim ‘First do no harm,‘ tolerance of adverse reactions to products given to healthy persons – especially healthy infants – is substantially lower than to products administered to persons who are already sick. This lower risk tolerance for vaccines translate into a need to investigate the possible causes of much rarer adverse events following vaccinations than would be acceptable for other pharmaceutical products. For example, events occurring at ~⅒⁵-⅒⁶ doses like acute encephalopathy after whole cell pertussis vaccine, Guillain-Barré syndrome (GBS) after swine in influenza vaccine and oral polio vaccine-associated paralytic polio (VAPP) are of concern for vaccines while side effects are essentially universal for cancer chemotherapy and 10-30% for persons on high dose aspirin therapy experience gastro-intestinal symptoms. […] there are several factors directly related to the vaccine community, due either to our action or inaction, that may have contributed to the current unsatisfactory situation. […] The cost and the difficulty of studying events increase with their rarity, however. […] in countries where immunizations are mandated, increasing the availability of philosophical exemptions may provide a ‘relief valve‘. In such situations, close monitoring the risk of vaccine-preventable disease in unimmunized exempters and their subsequent transmission to the larger community may help dissuade others. […] Improved vaccine risk communications, especially via a shift from traditional paternalistic to a shared decision making model, can help produce more informed consumers.”
Chen, R. T. (1999). Vaccine risks: real, perceived and unknown. Vaccine, 17, S41-S46. DOI: 10.1016/S0264-410X(99)00292-3. https://doi.org/10.1016/S0264-410X(99)00292-3
- (Chen, 1999c):
“We know that the sample size of even the largest pre-licensure trials, in the low thousand or tens of thousands, are calculated primarily based on efficacy rather than safety considerations. While such trials have advantages in their ability to assess causality of vaccine adverse events due to their experimental design, they are limited in their ability to provide data on rare, delayed, or reactions in subpopulations. Furthermore, the lack of standardization of case definitions for various adverse events (e.g. fever, fussiness) in such trials limit our ability to interpret and use these ‘safety‘ data. Due to these limitations, the ‘mantra‘ has been to rely on post-marketing surveillance to detect rare serious problems. Yet this has been more an aspiration than reality. […] Similarly, current knowledge and research capability about rare vaccine risks is incomplete and limited, as noted in extensive reviews in early 1990‘s by the Institute of Medicine (IOM) in the United States. Two-third of the 76 vaccine adverse events evaluated by the IOM were found to have either no or inadequate evidence to assess for or against a vaccine cause. […] as planes developed from propellers to jets, then jumbo jets and Concorde, the technology for improving aviation safety also evolved. Yes, in the event of an unfortunate crash, millions may have flown without difficulties, yet a careful investigation is still launched immediately to better understand this specific exception to the rule. Whatever changes needed, be it hardware, software, or policy, can then be identified and improved via systematic feedback. […] While much investment may have been made in the hardware and software in the vaccine development and vaccine production, much of the surveillance tools for vaccine safety remains relatively primitive. Important strides have been made in developing newer large-linked databases for active surveillance of vaccine safety. Yet nowhere globally are such systems stably funded or fully operational. Imagine having radar to avoid plane crashes and not using them, or having them but without enough staff to monitor the radar screen. […] the US vaccine excise tax has been jealously guarded by certain interests for use for injury compensation only, but not for improving understanding of vaccine safety as intended by its sponsor, Senator Paula Hawkins”
Chen, R. T. (1999). Vaccine risks: real, perceived and unknown. Vaccine, 17, S41-S46. DOI: 10.1016/S0264-410X(99)00292-3. https://doi.org/10.1016/S0264-410X(99)00292-3
- (Chen, 1999d):
“As the incidence of vaccine-preventable diseases is reduced by increasing coverage with an efficacious vaccine, vaccine adverse events, both those caused by vaccines (i.e. true adverse reactions) and those associated with vaccination only by coincidence, become increasingly frequent (Fig. 1). The number of both types of reports to the Vaccine Adverse Event Reporting System (VAERS) in the United States, approximately 11,000/year, now exceeds the reported incidence of most vaccine-preventable childhood diseases combined (Table 1).”
Chen, R. T. (1999). Vaccine risks: real, perceived and unknown. Vaccine, 17, S41-S46. DOI: 10.1016/S0264-410X(99)00292-3. https://doi.org/10.1016/S0264-410X(99)00292-3
- (Chen et al., 2001):
“Most national vaccine safety monitoring systems rely on some type of passive surveillance (also called ‘spontaneous reporting‘) system, such as the Vaccine Adverse Event Reporting System (VAERS) in the United States, to detect signals of potential safety concerns after licensure. For example, VAERS was the source of the signals for recent studies of Guillain-Barré syndrome (GBS) that occurred after administration of influenza vaccine and of intussusception that occurred after administration of rotavirus vaccine. However, these signals were detected within VAERS to a large extent because both GBS and intussusception are relatively unusual, yet specific, medical events. Most of the approximately 11,000 VAERS reports received annually are not so specific, however. Because serious vaccine adverse events are rare, most reporters to VAERS are making such a report for the first time. In contrast to clinical trials, in which the data collection process is standardized, VAERS reports are extremely heterogeneous with regard to clinical evaluation. Thus, analysis of VAERS reports, with their hodgepodge of symptoms, signs, laboratory results, and diagnoses, can be extremely difficult.
Another major factor that permitted the detection by VAERS of a possible increase in GBS after influenza vaccination was the manner in which the vaccine was administered. Influenza vaccine is usually administered alone, so there is little confusion about which vaccine may have caused the ensuing GBS (i.e., the specificity of exposure). Unfortunately, this is the exception rather than the rule for most VAERS reports.
First, the scientific advance of vaccine safety has been hindered by a lack of standard case definitions. Unlike vaccine efficacy, which can be measured by examination of the differential incidence rate of a single outcome with its case definition (i.e., the vaccine-preventable disease), safety cannot be measured directly. Safety can only be inferred indirectly from the absence of multiple likely adverse events, each measured separately in the trial. Hitherto, the absence of standard case definitions for vaccine adverse events has prevented comparison of vaccine safety data across clinical trials—even across the diphtheria-tetanus–acellular pertussis vaccine (DTPa) trials.
Because infectious disease epidemiologists have had the greatest interest in developing new vaccines, historically the epidemiologists that serve on most such boards and committees have come from this group. Because serious adverse events are generally rare, however, the expertise necessary to interpret them lies more in the area of chronic or ‘rare‘ disease epidemiology. In the case of rotavirus vaccine and intussusception, a reviewer with such expertise might have noticed that 4 of 5 intussusception cases seen among 10,054 vaccine recipients occurred within 2 weeks of vaccination, whereas the single case seen among the 4633 placebo recipients occurred several weeks after vaccination. A binomial test for randomness would have shown close to borderline statistical significance, instead of the nonsignificant result yielded by the x2 test actually used by the researchers.
Furthermore, the advent of Web-enabled standardized data collection raises the question of whether large, simple prelicensure safety trials might now be possible for vaccines, as were done for use of ibuprofen to treat children.
Since 1990, the CDC has organized the Vaccine Safety Datalink (VSD) project to enable scientifically rigorous studies of vaccine safety to be conducted. Unfortunately, the VSD study population constitutes only 2% of the US population. It is therefore not large or diverse enough to answer many vaccine safety questions in a timely manner. For example, the immunization schedule and the specific vaccines used in the 4 health maintenance organizations in the VSD are more homogeneous than those used in the US population in general.
Unlike most public health surveillance systems, which target a single exposure (e.g., lead) or a single disease outcome associated with a specific case definition (e.g., measles, salmonellosis), VAERS must conduct surveillance on multiple vaccine exposures and multiple disease outcomes (most without case definitions). In practical terms, this means hundreds of cross-tabulations in computer printouts that are inches deep and must be reviewed regularly by a human being for a possible signal. This is a process fraught with the risk that signals will be missed, because it is highly dependent on alertness and the experience of the reviewer. Not surprisingly, some VAERS signals arise as much from the persistence of a reporter as from prospective data review.
Even with large linked data sets such as the VSD and sophisticated analyses, however, it might not be possible to control adequately for ‘confounding by contraindication‘. Children who are less healthy are less likely to receive vaccines in a timely manner. Such children are also at increased risk for poor medical outcome. This confounding is, in essence, the mirror image of the ‘healthy worker‘ effect in epidemiology.
it may be possible to organize such a large, simple safety trial between licensure and mass adoption of a vaccine.
some risk is inevitably associated with vaccination. The National Vaccine Injury Compensation Program has been put in place to compensate those who are injured as a result of vaccination when, despite our best human efforts before or after licensure, some unexpected adverse events occur.”
Chen, R. T., Pool, V., Takahashi, H., Weniger, B. G., & Patel, B. (2001). Combination vaccines: postlicensure safety evaluation. Clinical infectious diseases, 33(Supplement_4), S327-S333. DOI: 10.1086/322569. https://doi.org/10.1086/322569
- (Children's Hospital of Philadelphia, 2025):
Children‘s Hospital of Philadelphia. (2025). Vaccine Safety References. Retrieved October, 2025, from https://www.chop.edu/vaccine-education-center/vaccine-safety/vaccine-safety-references
- (Colgrove, 2007):
“The most successful of the new products was a preparation against diphtheria called toxin-antitoxin, which became the second immunizing procedure to become commonplace. The vaccine was developed in the Bureau of Laboratories of the New York City Department of Health, whose director, William Hallock Park, conducted a pioneering series of trials beginning in 1913, first on children in the city’s orphanages and institutions, and then in the public school system. […] Since there was no systematic surveillance of immunization coverage levels [in the early 1900s], it is impossible to determine vaccination rates with any certainty, but special surveys provide some indications of moderate to high acceptance. In the late 1930s, for example, a survey in New York City found that about two-thirds of parents had had their children immunized against diphtheria. “
Colgrove, J. (2007). Immunity for the people: the challenge of achieving high vaccine coverage in American history. Public health reports, 122(2), 248-257. DOI: 10.1177/003335490712200215. https://doi.org/10.1177/003335490712200215 ; Recommended: https://journals.sagepub.com/doi/pdf/10.1177/003335490712200215
- (Cowling et al., 2012):
“We randomized 115 children to trivalent inactivated influenza vaccine (TIV) or placebo. Over the following 9 months, TIV recipients had an increased risk of virologically-confirmed non-influenza infections (relative risk: 4.40; 95% confidence interval: 1.31-14.8). Being protected against influenza, TIV recipients may lack temporary non-specific immunity that protected against other respiratory viruses.”
Cowling, B. J., Fang, V. J., Nishiura, H., Chan, K. H., Ng, S., Ip, D. K., … & Peiris, J. M. (2012). Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clinical Infectious Diseases, 54(12), 1778-1783. DOI: 10.1093/cid/cis307. https://doi.org/10.1093/cid/cis307 ; Recommended: https://academic.oup.com/cid/article-pdf/54/12/1778/17349931/cis307.pdf
- (Chua et al., 2021):
Chua, G. T., Kwan, M. Y. W., Chui, C. S., Smith, R. D., Cheung, E. C. L., Tian, T., … & Ip, P. (2021). Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clinical Infectious Diseases. DOI: 10.1093/cid/ciab989. https://doi.org/10.1093/cid/ciab989
- (Conis, 2019):
“The modern era of vaccination was heralded with the licensure of the first 2 measles vaccines in 1963.”
Conis, E. (2019). Measles and the modern history of vaccination. Public Health Reports, 134(2), 118-125. DOI: 10.1177/0033354919826558. https://doi.org/10.1177/0033354919826558 ; Recommended: https://journals.sagepub.com/doi/reader/10.1177/0033354919826558
- (Cutler et al., 2006):
Cutler, D., Deaton, A., & Lleras-Muney, A. (2006). The determinants of mortality. Journal of economic perspectives, 20(3), 97-120. DOI: 10.1257/jep.20.3.97. https://doi.org/10.1257/jep.20.3.97 ; Recommended: https://pubs.aeaweb.org/doi/pdfplus/10.1257/jep.20.3.97
- (Cutler & Miller, 2005):
Cutler, D., & Miller, G. (2005). The role of public health improvements in health advances: the twentieth-century United States. Demography, 42(1), 1-22. DOI: 10.1353/dem.2005.0002. https://doi.org/10.1353/dem.2005.0002 ; Recommended: https://link.springer.com/content/pdf/10.1353/dem.2005.0002.pdf
- (Delany et al., 2014):
“all agonists of innate receptors are potentially toxic and must be administered in a way that optimizes adjuvanticity but reduces local and systemic reactogenicity. These two characteristics of adjuvants are likely intrinsically linked and must be carefully balanced.”
Delany, I., Rappuoli, R., & De Gregorio, E. (2014). Vaccines for the 21st century. EMBO molecular medicine, 6(6), 708-720. DOI: 10.1002/emmm.201403876. https://doi.org/10.1002/emmm.201403876 ; Recommended: https://www.embopress.org/doi/pdf/10.1002/emmm.201403876
- (DeStefano et al., 2001):
“Vaccines containing the Urabe strain of mumps vaccine have been shown to be associated with an increased risk of aseptic meningitis.”
DeStefano, F., & Vaccine Safety Datalink Research Group. (2001). The vaccine safety datalink project. Pharmacoepidemiology and drug safety, 10(5), 403-406. DOI: 10.1002/pds.613. https://doi.org/10.1002/pds.613
- (DeStefano et al., 2001b):
“There are several inherent limitations of pre-licensure clinical trials including sample size, duration of follow-up, and population heterogeneity. As a result, postlicensure (also called postmarketing) evaluation of safety once vaccines are given to millions of persons is needed to evaluate rare, delayed, or unusual reactions. […] Historically, postlicensure monitoring in most countries, including the US, has relied on passive surveillance systems such as the VAERS. Because of the methodological limitations in passive surveillance for adverse events from drugs, during the 1980s pharmacoepidemiologists began turning to large databases that link computerized pharmacy prescription (and later, immunization) and medical outcome records. […] As the databases are usually generated in the routine administration of such programs and do not require completion of a vaccine adverse event reporting form, the problems of under-reporting or recall bias are reduced. Because these programs have enrollees numbering from thousands to millions, large populations can be examined for relatively infrequent adverse events. Denominator data on doses given and the ready availability of appropriate comparison groups also make these databases ideal for studying vaccine safety.”
DeStefano, F., & Vaccine Safety Datalink Research Group. (2001). The vaccine safety datalink project. Pharmacoepidemiology and drug safety, 10(5), 403-406. DOI: 10.1002/pds.613. https://doi.org/10.1002/pds.613
- (Di Pasquale et al., 2016):
Di Pasquale, A., Bonanni, P., Garçon, N., Stanberry, L. R., El-Hodhod, M., & Da Silva, F. T. (2016). Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine, 34(52), 6672-6680. DOI: 10.1016/j.vaccine.2016.10.039. https://doi.org/10.1016/j.vaccine.2016.10.039 ; Recommended: https://www.sciencedirect.com/science/article/pii/S0264410X16309744/pdfft?md5=0a7779a6640ad03641146557b593b8d1&pid=1-s2.0-S0264410X16309744-main.pdf
- (Ellenberg & Chen, 1997):
“In the late 1970s and early 1980s substantial public attention in the United States as well as in other developed countries was given to the safety of whole-cell pertussis vaccines. A few parents who believed their children had been seriously injured as a result of vaccination brought their concerns to the public through the media. Negative publicity about adverse events in Japan and the United Kingdom led to precipitous declines in vaccine coverage, with the consequent return of epidemic pertussis disease. A similar disease upsurge was observed in Sweden following discontinuation of pertussis vaccination in that country because of concerns about the efficacy of the vaccine in use there as well as safety concerns. In the United States, while public acceptance of pertussis vaccine generally remained high, numerous lawsuits were filed against vaccine manufacturers. This resulted in major increases in prices and decisions by several companies to discontinue manufacture of pertussis vaccines,10 resulting in temporary shortages. These events contributed to the passage of the National Childhood Vaccine Injury Act (NCVIA) in 1986.”
Ellenberg, S. S., & Chen, R. T. (1997). The complicated task of monitoring vaccine safety. Public Health Reports, 112(1), 10. https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/pdf/pubhealthrep00042-0012.pdf
- (Ellenberg & Chen, 1997b):
“Beeler, Varricchio and Wise noted occasional instances of life-threatening thrombocytopenias following the administration of MMR vaccine,”
Ellenberg, S. S., & Chen, R. T. (1997). The complicated task of monitoring vaccine safety. Public Health Reports, 112(1), 10. https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/pdf/pubhealthrep00042-0012.pdf
- (Ellenberg & Chen, 1997c):
“CDC epidemiologists reviewed reports of fever, seizures, and hospitalizations following administration of a newly licensed combination of diphtheria, tetanus and acellular pertussis vaccine (DTaP). The rate of such reports was about one-third lower than the reporting rate following the standard DTP vaccine, consistent with-and confirming in the context of general practice-the safety findings of the pre-licensure clinical trials. […] One recent VSD investigation confirmed an association between seizures and DTP and MMR vaccinations by comparing vaccine exposures within specified time periods (one day for DTP, one week for MMR).”
Ellenberg, S. S., & Chen, R. T. (1997). The complicated task of monitoring vaccine safety. Public Health Reports, 112(1), 10. https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/pdf/pubhealthrep00042-0012.pdf
- (Ellenberg & Chen, 1997d):
“Concerns have been raised about reactions being exacerbated when vaccines are combined; while current experience does not suggest that there would be insurmountable safety problems with adding new vaccines to currently available combinations, the possibility of increased reactogenicity is well recognized.”
Ellenberg, S. S., & Chen, R. T. (1997). The complicated task of monitoring vaccine safety. Public Health Reports, 112(1), 10. https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/pdf/pubhealthrep00042-0012.pdf
- (Ellenberg & Chen, 1997e):
“We would all like such products to pose zero risk of adverse effects. Unfortunately, this goal is not achievable for any pharmacologically active product-if there is a beneficial effect, there will be some risk, however tiny, of an adverse effect.”
Ellenberg, S. S., & Chen, R. T. (1997). The complicated task of monitoring vaccine safety. Public Health Reports, 112(1), 10. https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1381831/pdf/pubhealthrep00042-0012.pdf
- (Ellenberg & Braun, 2002):
“While these vaccines protect against a variety of infectious diseases, they clearly do not protect against other adverse outcomes that affect children. Thus, vaccinated children remain susceptible to sudden infant death syndrome (SIDS), child-hood cancers, diabetes mellitus, mental retardation, developmental disorders, and other serious conditions that are diagnosed in childhood. Although the incidence of such disorders is rare, the numbers of children newly diagnosed with these conditions range from several hundred to thousands each year. Nearly all of these incident cases will have been preceded by one or more vaccinations, and in some cases, by only a day or two. The fact of such diagnoses cannot therefore be viewed as conclusive evidence of an adverse vaccine effect; but neither can the fact that we expect such events to follow vaccination be considered proof of non-vaccine causality in any individual case. When the time from vaccination to the adverse outcome is short, many who are not experienced in the review of such data will find it difficult to accept that the temporal association is not sufficient to establish causality.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2165/00002018-200225030-00001. https://doi.org/10.2165/00002018-200225030-00001
- (Ellenberg & Braun, 2002b):
“Although it may be reasonable to question whether pre-licensure trials ought to be large enough to detect (or rule out) risks of a given size in addition to detecting a given level of efficacy, there will always be a threshold beyond which risks are too small to be discovered until a vaccine is in widespread use.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2165/00002018-200225030-00001. https://doi.org/10.2165/00002018-200225030-00001
- (Ellenberg & Braun, 2002c):
“reporting is affected by the proximity of the event to vaccination. Events occurring weeks following vaccination are less likely to be reported than those occurring shortly after vaccination. […] it limits information on non-acute events whose onset after a causal exposure may be delayed. Many of the conditions for which there is public concern about possible connections with vaccination are of this type, for example, type 1 diabetes mellitus, autism, multiple sclerosis.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2165/00002018-200225030-00001. https://doi.org/10.2165/00002018-200225030-00001
- (Ellenberg & Braun, 2002d):
“Another example of an adverse event identified through VAERS was hair loss following vaccination. Prompted by a report from a concerned parent, FDA staff searched the VAERS database for similar cases and were somewhat surprised to find about 40 cases of alopecia that had been reported to VAERS over a period of years. The lag in detection may have been due at least in part to the fact that few, if any of these reports met the FDA definition of a serious adverse event (defined as death, hospitalisation, prolongation of hospitalisation, life-threatening illness or permanent disability) so they had not been a priority for review and follow-up. Although follow-up identified other possible causes in many cases (e.g. use of chemical hair treatments), there were several cases of ‘positive rechallenge‘: hair loss after vaccination, followed by regrowth and then hair loss again after the next vaccine dose. These cases provided a strong suggestion of a real, but clearly very rare, effect. Cases were reported in both children and adults; several different vaccines were involved but most cases included receipt of hepatitis B vaccine. Positive rechallenge represents a type of temporal relation that is particularly persuasive. Despite the absence of strong data supporting the other causality criteria, the high value usually placed on positive rechallenge data lends credibility to the potentially causal association between alopecia and vaccination.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2165/00002018-200225030-00001. https://doi.org/10.2165/00002018-200225030-00001
- (Ellenberg et al., 2005):
“Vaccines are different from most other pharmaceuticals in ways that influence safety considerations. They are administered to millions of healthy people every year, and, unlike any therapeutic product, many vaccines are mandated for entry into schools, pre-school programs, and day care programs by most states, as well as during military service. Vaccines are known to cause a small number of extremely rare but serious adverse outcomes, such as vaccine-associated paralytic polio and anaphylactic shock, but are suspected by some to be responsible for a much larger number and wide variety of serious and chronic health problems. It is often difficult to address such concerns effectively because, as discussed earlier, evaluating vaccine safety in the postlicensure setting is very complicated. With vaccines, as with other widely used products, some serious medical events will occur coincidentally after administration. Often it is impossible at that time to ascertain the likelihood of any causal connection with the vaccine; because such events occur in the absence as well as the presence of vaccines, causal effect is rarely demonstrable.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2105/AJPH.2004.039438. https://doi.org/10.2105/AJPH.2004.039438 ; Recommended: https://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2004.039438
- (Ellenberg et al., 2005b):
“Controlled trials conducted prior to licensure are designed to establish efficacy and assess relatively common adverse events, and they are usually far too small to detect rare, serious outcomes that could still affect large numbers of children each year. A trial of 5000 participants, equally divided between new vaccine and control groups, would have good power to detect a doubling of an adverse event that might occur in 1% of the population but would have virtually no power to detect a doubling of an event occurring in only 0.1% of the population. Such an adverse event would, however, affect as many as 4000 children a year in the United States alone. Also, the inclusion criteria in these trials are often narrow, excluding children with chronic or acute illness; broadening these criteria would allow some data to be obtained, in a controlled setting incorporating active monitoring for safety, with children who might be at increased risk of adverse events and ultimately would be part of the vaccine‘s target population.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2105/AJPH.2004.039438. https://doi.org/10.2105/AJPH.2004.039438 ; Recommended: https://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2004.039438
- (Ellenberg et al., 2005c):
“despite the inclusion of more than 1 million children in the Salk inactivated polio vaccine trial, postlicensure manufacturing and quality control problems led to the ‘Cutter incident.‘ In addition, contamination of early vaccine products with a macaque polyomavirus (SV-40) that was present in macaque kidney tissue used in the manufacture of inactivated polio vaccine was not recognized until years after the vaccines were put into use.”
Ellenberg, S. S., & Braun, M. M. (2002). Monitoring the safety of vaccines: assessing the risks. Drug Safety, 25(3), 145-152. DOI: 10.2105/AJPH.2004.039438. https://doi.org/10.2105/AJPH.2004.039438 ; Recommended: https://ajph.aphapublications.org/doi/pdf/10.2105/AJPH.2004.039438
- (Enriquez et al., 2005):
“Parents who refuse vaccinations reported less asthma and allergies in their unvaccinated children.”
Enriquez, R., Addington, W., Davis, F., Freels, S., Park, C. L., Hershow, R. C., & Persky, V. (2005). The relationship between vaccine refusal and self-report of atopic disease in children. Journal of allergy and clinical immunology, 115(4), 737-744. DOI: 10.1016/j.jaci.2004.12.1128. https://doi.org/10.1016/j.jaci.2004.12.1128
- (Feely et al., 1990):
Feely, J., Moriarty, S., & O‘Connor, P. (1990). Stimulating reporting of adverse drug reactions by using a fee. BMJ: British Medical Journal, 300(6716), 22. DOI: 10.1136/bmj.300.6716.22. https://doi.org/10.1136/bmj.300.6716.22 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1661889/pdf/bmj00160-0028.pdf
- (Fiebelkorn et al., 2016):
“We did not find compelling data to support a routine third dose of MMR vaccine.”
Fiebelkorn, A. P., Coleman, L. A., Belongia, E. A., Freeman, S. K., York, D., Bi, D., … & Beeler, J. (2016). Measles virus neutralizing antibody response, cell-mediated immunity, and immunoglobulin G antibody avidity before and after receipt of a third dose of measles, mumps, and rubella vaccine in young adults. The Journal of infectious diseases, 213(7), 1115-1123. DOI: 10.1093/infdis/jiv555. https://doi.org/10.1093/infdis/jiv555 ; Recommended: https://academic.oup.com/jid/article-pdf/213/7/1115/17410880/jiv555.pdf
- (Fulginiti et al., 1967):
Fulginiti, V. A., Eller, J. J., Downie, A. W., & Kempe, C. H. (1967). Altered reactivity to measles virus: atypical measles in children previously immunized with inactivated measles virus vaccines. Jama, 202(12), 1075-1080. DOI: 10.1001/jama.1967.03130250057008. https://doi.org/10.1001/jama.1967.03130250057008
- (Gatti & Montanari, 2016):
Gatti, A. M., & Montanari, S. (2016). New quality-control investigations on vaccines: micro-and nanocontamination. Int J Vaccines Vaccin, 4(1), 00072. DOI: 10.15406/ijvv.2017.04.00072. https://doi.org/10.15406/ijvv.2017.04.00072
- (Glanz et al., 2016):
Glanz, J. M., Newcomer, S. R., Jackson, M. L., Omer, S. B., Bednarczyk, R. A., Shoup, J. A., … & Sukumaran, L. (2016). White Paper on studying the safety of the childhood immunization schedule in the Vaccine Safety Datalink. Vaccine, 34, A1-A29. DOI: 10.1016/j.vaccine.2015.10.082. https://doi.org/10.1016/j.vaccine.2015.10.082
- (Goldman, 2013):
“Based on the prior record of safety of TIV and the fact that the pandemic A-H1N1 vaccine shared the same licensure and manufacturing processes as the seasonal TIV, the ACIP recommended for the 2009/2010 influenza season that pregnant women receive the pandemic inactivated A-H1N1-virus vaccine in addition to the seasonal TIV (both produced by five approved vaccine manufacturers) during any trimester of pregnancy.
Although there was an approximate fourfold (43%/11.3%) increase in the percentage of pregnant women vaccinated in 2009/2010 compared with 2008/2009, there was a 43.5-fold increase in fetal-loss reports – from 4 in 2008/2009 to 174 in 2009/2010. The report RR of 11.4 (95% CI: 4.2–30.8) of the 2009/2010 rate of 77.8 fetal-loss reports/1 million pregnant women vaccinated to the 2008/2009 report rate of 6.8 fetal-loss reports/1 million pregnant women vaccinated is statistically significant”
Goldman, G. S. (2013). Comparison of VAERS fetal-loss reports during three consecutive influenza seasons: Was there a synergistic fetal toxicity associated with the two-vaccine 2009/2010 season?. Human & experimental toxicology, 32(5), 464-475. DOI: 10.1177/0960327112455067. https://doi.org/10.1177/0960327112455067 ; Recommended: https://journals.sagepub.com/doi/reader/10.1177/0960327112455067
- (Goldman & Miller, 2023):
Goldman, G. S., & Miller, N. Z. (2023). Reaffirming a positive correlation between number of vaccine doses and infant mortality rates: A response to critics. Cureus, 15(2). DOI: 10.7759/cureus.34566. https://doi.org/10.7759/cureus.34566 ; Recommended: https://assets.cureus.com/uploads/original_article/pdf/134233/20230204-13155-1rklw73.pdf
- (Grove & Hetzel, 1968):
“Figure 19.—Death Rates for Measles: Death-registration States, 1900-32, and United States, 1933-60; (Rates per 100,000 population).”
Grove, R. D., & Hetzel, A. M. (1968). Vital statistics rates in the United States, 1940-1960 (No. 1677). US Government Printing Office. https://www.cdc.gov/nchs/data/vsus/vsrates1940_60.pdf ; Recommended: https://www.cdc.gov/nchs/data/vsus/vsrates1940_60.pdf#page=93
- (Grove & Hetzel, 1968b):
“Figure 18.—Death Rates for Diphtheria: Death-registration States, 1900-32, and United States, 1933-60; (Rates per 100,000 population).”
Grove, R. D., & Hetzel, A. M. (1968). Vital statistics rates in the United States, 1940-1960 (No. 1677). US Government Printing Office. https://www.cdc.gov/nchs/data/vsus/vsrates1940_60.pdf ; Recommended: https://www.cdc.gov/nchs/data/vsus/vsrates1940_60.pdf#page=92
- (Guyer et al., 2000):
“Vaccines against diphtheria, tetanus, and pertussis became available during the late 1920s but only widely used in routine pediatric practice after World War II. Thus vaccination does not account for the impressive declines in mortality seen in the first half of the century. […] For children older than 1 year of age, the overall decline in mortality experienced during the 20th century has been spectacular (Fig 8). In 1900 >3 in 100 children died between their first and 20th birthday; today, <2 in 1000 die. Nearly 85% of this decline took place before World War II, a period when few antibiotics or modern vaccines and medications were available.”
Guyer, B., Freedman, M. A., Strobino, D. M., & Sondik, E. J. (2000). Annual summary of vital statistics: trends in the health of Americans during the 20th century. Pediatrics, 106(6), 1307-1317. DOI: 10.1542/peds.106.6.1307. https://doi.org/10.1542/peds.106.6.1307
- (Hadinegoro et al, 2015):
“During year 3, hospitalization for severe dengue, as defined by the independent data monitoring committee criteria, occurred in 18 of 22,177 participants in the vaccine group and 6 of 11,089 participants in the control group.”
Hadinegoro, S. R., Arredondo-García, J. L., Capeding, M. R., Deseda, C., Chotpitayasunondh, T., Dietze, R., … & Saville, M. (2015). Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. New England Journal of Medicine, 373(13), 1195-1206. DOI: 10.1056/NEJMoa1506223. https://doi.org/10.1056/NEJMoa1506223 ; Recommended: https://www.nejm.org/doi/pdf/10.1056/NEJMoa1506223
- (Hall et al., 2021):
“HBV is transmitted by parenteral or mucosal exposure to HBsAg-positive body fluids from persons who have acute or chronic HBV infection.”
Hall, E., Wodi, A. P., Hamborsky, J., & Centers for Disease Control and Prevention (Eds.). (2021). The Pink Book: Epidemiology and prevention of vaccine-preventable diseases (14th ed.). Public Health Foundation. https://www.cdc.gov/pinkbook/hcp/table-of-contents/index.html
- (Hall et al., 2021b):
“HPV is transmitted through intimate, skin-to-skin contact with an infected person. Transmission is most common during vaginal, penile, anal, or oral sex.”
Hall, E., Wodi, A. P., Hamborsky, J., & Centers for Disease Control and Prevention (Eds.). (2021). The Pink Book: Epidemiology and prevention of vaccine-preventable diseases (14th ed.). Public Health Foundation. https://www.cdc.gov/pinkbook/hcp/table-of-contents/index.html
- (Hall et al., 2021c):
“Tetanus is not contagious from person-to-person.”
Hall, E., Wodi, A. P., Hamborsky, J., & Centers for Disease Control and Prevention (Eds.). (2021). The Pink Book: Epidemiology and prevention of vaccine-preventable diseases (14th ed.). Public Health Foundation. https://www.cdc.gov/pinkbook/hcp/table-of-contents/index.html
- (Hammond et al., 2022):
Hammond, N. E., Zampieri, F. G., Di Tanna, G. L., Garside, T., Adigbli, D., Cavalcanti, A. B., … & Delaney, A. (2022). Balanced crystalloids versus saline in critically ill adults—a systematic review with meta-analysis. NEJM evidence, 1(2), EVIDoa2100010. DOI: 10.1056/EVIDoa2100010. https://doi.org/10.1056/EVIDoa2100010 ; Recommended: https://evidence.nejm.org/doi/pdf/10.1056/EVIDoa2100010
- (Hasford et al., 2002):
“Even serious suspected adverse reactions are only reported in 5–15% of all incident cases.”
Hasford, J., Goettler, M., Munter, K. H., & Müller-Oerlinghausen, B. (2002). Physicians‘ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. Journal of clinical epidemiology, 55(9), 945-950. DOI: 10.1016/S0895-4356(02)00450-X. https://doi.org/10.1016/S0895-4356(02)00450-X
- (Hearing Before the Subcommittee on Human Rights and Wellness, 2004):
“Mr. Burton. Has thimerosal ever really been tested? Has thimerosal ever been tested by our health agencies? Mr. Egan. Only in those early tests that you know of that were done by Lily. Mr. Burton. When was that? That was done in 1929. Let‘s followup on that. In 1929, they tested this on 27 people that were dying of meningitis. All of those people died of meningitis, so they said there was no correlation between their death and the mercury in the vaccines. That is the only test that‘s ever been done on thimerosal that I know of. Can you think of any other? Mr. Egan. No, in people, no. Except for accidental exposures over time.”
Hearing Before the Subcommittee on Human Rights and Wellness: U.S. Committee on Government Reform, 108th Congress (2004) (Testimony of William Egan, Acting Director, U.S. FDA). https://www.congress.gov/108/chrg/CHRG-108hhrg98046/CHRG-108hhrg98046.pdf
- (Henderson, 2011):
Henderson, D. A. (2011). The eradication of smallpox–an overview of the past, present, and future. Vaccine, 29, D7-D9. DOI: 10.1016/j.vaccine.2011.06.080. https://doi.org/10.1016/j.vaccine.2011.06.080
- (Herve et al., 2019):
Hervé, C., Laupèze, B., Del Giudice, G., Didierlaurent, A. M., & Tavares Da Silva, F. (2019). The how’s and what’s of vaccine reactogenicity. npj Vaccines, 4(1), 39. DOI: 10.1038/s41541-019-0132-6. https://doi.org/10.1038/s41541-019-0132-6 ; Recommended: https://www.nature.com/articles/s41541-019-0132-6.pdf
- (Higgins et al., 2016):
“receipt of DTP may be associated with an increase in all cause mortality. Although efforts should be made to ensure that all children are immunised on schedule with BCG, DTP, and MCV, randomised trials are needed to compare the effects of different sequences.”
Higgins, J. P., Soares-Weiser, K., López-López, J. A., Kakourou, A., Chaplin, K., Christensen, H., … & Reingold, A. L. (2016). Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. bmj, 355. DOI: 10.1136/bmj.i5170. https://doi.org/10.1136/bmj.i5170 ; Recommended: https://www.bmj.com/content/355/bmj.i5170.full.pdf
- (Hoberman et al., 2003):
“During the second year of the study the rate of hospitalization was actually higher in the vaccine group than in the placebo group.”
Hoberman, A., Greenberg, D. P., Paradise, J. L., Rockette, H. E., Lave, J. R., Kearney, D. H., … & Kerr, J. D. (2003). Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. Jama, 290(12), 1608-1616. DOI: 10.1001/jama.290.12.1608. https://doi.org/10.1001/jama.290.12.1608
- (Hodel et al., 2024):
Hodel, K. V. S., Fiuza, B. S. D., Conceição, R. S., Aleluia, A. C. M., Pitanga, T. N., Fonseca, L. M. D. S., … & Machado, B. A. S. (2024). Pharmacovigilance in vaccines: importance, main aspects, perspectives, and challenges—a narrative review. Pharmaceuticals, 17(6), 807. DOI: 10.3390/ph17060807. https://doi.org/10.3390/ph17060807 ; Recommended: https://www.mdpi.com/1424-8247/17/6/807/pdf?version=1719319314
- (Hooker & Miller, 2020):
Hooker, B. S., & Miller, N. Z. (2020). Analysis of health outcomes in vaccinated and unvaccinated children: Developmental delays, asthma, ear infections and gastrointestinal disorders. SAGE Open Medicine, 8, 2050312120925344. DOI: 10.1177/2050312120925344. https://doi.org/10.1177/2050312120925344 ; Recommended: https://journals.sagepub.com/doi/pdf/10.1177/2050312120925344
- (Hooker & Miller, 2021):
Hooker, B. S., & Miller, N. Z. (2021). Health effects in vaccinated versus unvaccinated children, with covariates for breastfeeding status and type of birth. Journal of Translational Science, 7, 1-11. DOI: 10.15761/JTS.1000459. https://doi.org/10.15761/JTS.1000459 ; Recommended: https://www.oatext.com/pdf/JTS-7-459.pdf
- (Institute of Medicine, 2002):
Institute of Medicine. (2002). Immunization Safety Review: Multiple Immunizations and Immune Dysfunction. DOI: 10.17226/10306. https://doi.org/10.17226/10306 ; Recommended: https://doi.org/10.17226/10306
- (Institute of Medicine, 2013):
“Vaccinations—like all medical procedures—are neither 100 percent free of risk nor 100 percent effective. Vaccines, in rare cases, can cause illness. Most children who experience an adverse reaction to immunization have a preexisting susceptibility. Some predispositions may be detectable prior to vaccination; others, at least with current technology and practice, are not.
No studies have compared the differences in health outcomes that some stakeholders questioned between entirely unimmunized populations of children and fully immunized children. Experts who addressed the committee pointed not to a body of evidence that had been overlooked but rather to the fact that existing research has not been designed to test the entire immunization schedule.
The committee believes that although the available evidence is reassuring, studies designed to examine the long-term effects of the cumulative number of vaccines or other aspects of the immunization schedule have not been conducted.
The conduct of an RCT would require thousands of participants to be of sufficient size to answer questions about the outcomes of different immunization schedules, and the study would have to span at least 6 to 10 years, meaning that it would likely cost the nation tens of millions of dollars.
The committee acknowledges that large-scale, long-term studies of infants through adulthood would be informative for evaluating health outcomes associated with immunization.
The most feasible approach to studying the safety of the childhood immunization schedule is through analyses of data obtained by VSD. VSD is a collaborative effort between CDC and 9 managed care organizations that maintain a large database of linked data for monitoring immunization safety and studying potential rare and serious adverse events. VSD member sites include data for more than 9 million children and adults receiving vaccinations on a variety of immunization schedules. However, children who are vaccinated on alternative schedules (including those who are not vaccinated) may differ in meaningful ways. Although this confounding can be minimized through matching and controlling for variations, differences in nonrandomly constructed cohorts cannot be fully accounted for by the use of these data.
The committee discussed several potential modifications that could be introduced into this system that would enable new analyses of the key research questions (Box S-2), including collection of additional data on the participants. The committee found that secondary analyses within VSD would advance knowledge of the safety of the immunization schedule and identified enhancements to improve the data in VSD.
One potential enhancement to VSD would be to collect additional demographic and, possibly, family history data for current participants. Basic information on vaccination history, child gender, race/ethnicity, and birth status (e.g., gestational age or birth weight) could be systematically collected for all participants. New approaches to the collection of additional data on a family history of allergies, autoimmune disorders, neurological disorders, and the like should be considered. These data would permit analyses of the fourth research question (about potentially susceptible sub-populations) that cannot be readily conducted at this time.
Most vaccine-related research focuses on the outcomes of single immunizations or combinations of vaccines administered at a single visit. Although each new vaccine is evaluated in the context of the overall immunization schedule that existed at the time of review of that vaccine, elements of the schedule are not evaluated once it is adjusted to accommodate a new vaccine. Thus, key elements of the entire schedule—the number, frequency, timing, order, and age at administration of vaccines—have not been systematically examined in research studies.
Finally, the committee found that evidence assessing outcomes in sub-populations of children who may be potentially susceptible to adverse reactions to vaccines (such as children with a family history of autoimmune disease or allergies or children born prematurely) was limited and is characterized by uncertainty about the definition of populations of interest and definitions of exposures and outcomes.”
Institute of Medicine. (2013). The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. DOI: 10.17226/13563. https://doi.org/10.17226/13563
- (Institute of Medicine, 2013b):
“In summary, few studies have comprehensively assessed the association between the entire immunization schedule or variations in the overall schedule and categories of health outcomes. […] studies designed to examine the long-term effects of the cumulative number of vaccines or other aspects of the immunization schedule have not been conducted.”
Institute of Medicine. (2013). The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. DOI: 10.17226/13563. https://doi.org/10.17226/13563
- (Jablonowski & Hooker, 2024):
“Following from Table 1, each additional vaccine more than doubles the diseases diagnosed.” gJablonowski, K., & Hooker, B. (2024). Adverse outcomes are increased with exposure to added combinations of infant vaccines. International Journal of Vaccine Theory, Practice, and Research, 3(2), 1103-1111. DOI: 10.56098/xfzkf650. https://doi.org/10.56098/xfzkf650
- (Jablonowski & Hooker, 2025):
“Children vaccinated in their 2nd month of life were between 29%-74% (depending on vaccine) more likely to die in their 3rd month, between 28%-74% if black, and between 52%-98% if female.” gJablonowski, K., & Hooker, B. (2025). Increased Mortality Associated with 2-Month Old Infant Vaccinations. DOI: 10.5281/zenodo.18262930. https://doi.org/10.5281/zenodo.18262930
- (Jefferson et al., 2010):
“Influenza vaccines have a modest effect in reducing influenza symptoms and working days lost. There is no evidence that they affect complications, such as pneumonia, or transmission.”
Jefferson, T., Di Pietrantonj, C., Rivetti, A., Bawazeer, G. A., Al-Ansary, L. A., & Ferroni, E. (2010). Vaccines for preventing influenza in healthy adults. Cochrane database of systematic reviews. DOI: 10.1002/14651858.CD001269.pub4. https://doi.org/10.1002/14651858.CD001269.pub4 ; Recommended: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001269.pub4/epdf/full/en
- (Kass, 1971):
“the overall decline in deaths from tuberculosis was not altered measurably by the discovery of the tubercle bacillus, the advent of the tuberculin test, the appearance of BCG vaccination, the widespread use of mass screening, the intensive anti-tuberculosis campaigns, or the discovery of streptomycin. Only the advent of isoniazid changed the mortality patterns, and by then the rate of tuberculosis had fallen to but a small fraction of its levels 100 years earlier. […] This decline in rates of certain disorders, correlated roughly with improving socioeconomic circumstances, is merely the most important happening in the history of the health of man, yet we have only the vaguest and most general notions about how it happened and by what mechanisms socioeconomic improvement and decreased rates of certain diseases run in parallel.”
Kass, E. H. (1971). Infectious diseases and social change. The Journal of Infectious Diseases, 123(1), 110-114. DOI: 10.1093/infdis/123.1.110. https://doi.org/10.1093/infdis/123.1.110
- (Kim et al., 1969):
“It seems clear that infants who received this vaccine were not protected against natural infection and also, when they became naturally infected their illness was more severe. […] These findings together suggest that RS virus illness in infants is an immunologic phenomenon wherein the virus and serum antibody interact to produce severe illness.”
Kim, H. W., Canchola, J. G., Brandt, C. D., Pyles, G., Chanock, R. M., Jensen, K., & Parrott, R. H. (1969). Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. American journal of epidemiology, 89(4), 422-434. DOI: 10.1093/oxfordjournals.aje.a120955. https://doi.org/10.1093/oxfordjournals.aje.a120955
- (Kimura & Kuno-Sakai, 1990):
“After the temporary suspension of pertussis vaccine followed by a rapid drop in pertussis vaccine acceptance rates, there were epidemics of pertussis, with a peak in 1979. Since 1979, there has been a sustained fall in reported pertussis cases. […] Reported cases of pertussis and deaths (A) and age-specific incidence of reported pertussis (B).”
Kimura, M., & Kuno-Sakai, H. (1990). Developments in pertussis immunisation in Japan. The Lancet, 336(8706), 30-32. DOI: 10.1016/0140-6736(90)91530-N. https://doi.org/10.1016/0140-6736(90)91530-N
- (Klein, 2014):
“In 1948, the pertussis vaccine was combined with diphtheria and tetanus toxoids and became a diphtheria whole cell pertussis and tetanus toxoid (DTP) vaccine. “
Klein, N. P. (2014). Licensed pertussis vaccines in the United States: history and current state. Human vaccines & immunotherapeutics, 10(9), 2684-2690. DOI: 10.4161/hv.29576. https://doi.org/10.4161/hv.29576 ; Recommended: https://www.tandfonline.com/doi/pdf/10.4161/hv.29576
- (Lam et al., 2024):
Lam, N., Lee, Y., & Farber, D. L. (2024). A guide to adaptive immune memory. Nature Reviews Immunology, 24(11), 810-829. DOI: 10.1038/s41577-024-01040-6. https://doi.org/10.1038/s41577-024-01040-6 ; Recommended: https://www.nature.com/articles/s41577-024-01040-6.pdf
- (Lamerato et al., 2020):
“This study found that exposure to vaccination was independently associated with an overall 2.5-fold increase in the likelihood of developing a chronic health condition, when compared to children unexposed to vaccination. This association was primarily driven by asthma, atopic disease, eczema, autoimmune disease and neurodevelopmental disorders. This suggests that in certain children, exposure to vaccination may increase the likelihood of developing a chronic health condition, particularly for one of these conditions.”
Lamerato, L., Chatfield, A., Tang, A., & Zervos, M. (2020). Impact of Childhood Vaccination on Short and Long-Term Chronic Health Outcomes in Children: A Birth Cohort Study [Unpublished manuscript]. Henry Ford Health System. https://www.hsgac.senate.gov/wp-content/uploads/Entered-into-hearing-record-Impact-of-Childhood-Vaccination-on-Short-and-Long-Term-Chronic-Health-Outcomes-in-Children-A-Birth-Cohort-Study.pdf
- (Le Vu et al., 2022):
Le Vu, S., Bertrand, M., Jabagi, M. J., Botton, J., Drouin, J., Baricault, B., … & Zureik, M. (2022). Age and sex-specific risks of myocarditis and pericarditis following Covid-19 messenger RNA vaccines. Nature communications, 13(1), 1-9. DOI: 10.1038/s41467-022-31401-5. https://doi.org/10.1038/s41467-022-31401-5
- (Lazarus et al., 2010):
“Fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed. Barriers to reporting include a lack of clinician awareness, uncertainty about when and what to report, as well as the burdens of reporting: reporting is not part of clinicians’ usual workflow, takes time, and is duplicative.”
Lazarus, R., Klompas, M., & Bernstein, S. (2010). Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP: VAERS). The Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. https://digital.ahrq.gov/sites/default/files/docs/publication/r18hs017045-lazarus-final-report-2011.pdf
- (Liang et al., 2010):
“Severe adverse effects occurred in 69 (0·6%, 0·5–0·8) recipients of vaccine compared with one recipient (0·1%, 0–0·2) of placebo.”
Liang, X. F., Wang, H. Q., Wang, J. Z., Fang, H. H., Wu, J., Zhu, F. C., … & Wang, Y. (2010). Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. The Lancet, 375(9708), 56-66. DOI: 10.1016/S0140-6736(09)62003-1. https://doi.org/10.1016/S0140-6736(09)62003-1
- (Lyons-Weiler & Thomas, 2020):
Lyons-Weiler, J., & Thomas, P. (2020). Relative Incidence of Office Visits and Cumulative Rates of Billed Diagnoses Along the Axis of Vaccination. International Journal of Environmental Research and Public Health, 17(22), 8674. DOI: 10.3390/ijerph17228674. https://doi.org/10.3390/ijerph17228674
- (Maglione et al., 2014):
“Controlled trials often have insufficient sample size to identify rare AEs and do not have extended follow-up to identify long-term sequelae. In addition, trials may purposely exclude subjects who could be more susceptible to AEs. For this reason, any comprehensive review of vaccine safety must include post-licensure studies, but these also have limitations. Large epidemiologic studies sometimes include any available formulation of vaccines against a particular disease and may not stratify results by dosage or formulation. For example, the relationship between the ‘seasonal influenza vaccine‘ and an AE could be studied over several years of data without considering the changes in formulation over the seasons or differentiating between live or inactive vaccine. In addition, people who avoid vaccinations (whether purposely or not) may differ from those who receive vaccinations in terms of race, gender, age, socioeconomic status, and preexisting medical conditions, and these differences may be associated with health outcomes. Observational studies may attempt to control for such potential confounders by using matched cohorts or multivariate regression analysis; still, some factors such as environmental exposures may be unmeasured or challenging to adequately control for.
The self-controlled case series was developed specifically to assess the safety of vaccines; this method eliminates confounding by all time-independent variables by using cases as their own controls and predefined ‘time windows‘ before and after vaccination. This design has been used to study purpura, febrile seizures, intussusception, and autism in children. However, the assumption of no temporal shifts in this model is difficult to justify in very young children because any patient characteristics that change with time will not be adequately controlled for.”
Maglione, M. A., Das, L., Raaen, L., Smith, A., Chari, R., Newberry, S., … & Gidengil, C. (2014). Safety of vaccines used for routine immunization of US children: a systematic review. Pediatrics, 134(2), 325-337. DOI: 10.1542/peds.2014-1079. https://doi.org/10.1542/peds.2014-1079
- (Maglione et al., 2014b):
“Evidence was found for an association of several serious AEs with vaccines; however, these events were extremely rare: absolute risk is low. For example, strength of evidence is moderate for association of vaccines against rotavirus with intussusception.”
Maglione, M. A., Das, L., Raaen, L., Smith, A., Chari, R., Newberry, S., … & Gidengil, C. (2014). Safety of vaccines used for routine immunization of US children: a systematic review. Pediatrics, 134(2), 325-337. DOI: 10.1542/peds.2014-1079. https://doi.org/10.1542/peds.2014-1079
- (Mahmood et al., 2014):
Mahmood, M., Lee, W., Mamertino, M., Saget, C., Malgouyres, C., & Giovanzana, M. (2014). Growth patterns in developing countries. World of Work Report, 2014(1), 17-32. DOI: 10.1002/wow3.44. https://doi.org/10.1002/wow3.44 ; Recommended: https://onlinelibrary.wiley.com/doi/pdf/10.1002/wow3.44
- (Mawson & Jacob, 2025):
Mawson, A. R., & Jacob, B. I. N. U. (2025). Vaccination and neurodevelopmental disorders: A study of nine-year-old children enrolled in Medicaid. Science, Public Health Policy & the Law, 2019-2025. https://publichealthpolicyjournal.com/vaccination-and-neurodevelopmental-disorders-a-study-of-nine-year-old-children-enrolled-in-medicaid/ ; Recommended: https://publichealthpolicyjournal.com/wp-content/uploads/2025/01/Mawson_Jacob_SciencePublicHealthPolicyAndTheLaw_v6.2019-2025.Jan_2025.pdf
- (Mawson et al., 2017):
Mawson, A. R., Ray, B. D., Bhuiyan, A. R., & Jacob, B. (2017). Pilot comparative study on the health of vaccinated and unvaccinated 6-to 12-year-old US children. J Transl Sci, 3(3), 1-12. DOI: 10.15761/JTS.1000186. https://doi.org/10.15761/JTS.1000186
- (McCarthy et al., 2017):
“Although there were few deaths, our results do not indicate a difference in risk of all-cause mortality among fully vaccinated versus undervaccinated children.”
McCarthy, N. L., Sukumaran, L., Newcomer, S., Glanz, J., Daley, M. F., McClure, D., … & Weintraub, E. (2017). Patterns of childhood immunization and all-cause mortality. Vaccine, 35(48), 6643-6648. DOI: 10.1016/j.vaccine.2017.10.034. https://doi.org/10.1016/j.vaccine.2017.10.034 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC6506838/pdf/nihms-1024630.pdf
- (McCormick et al., 2002):
McCormick, M. C., Wilson, C. B., & Stratton, K. (Eds.). (2002). Immunization safety review: multiple immunizations and immune dysfunction. https://www.ncbi.nlm.nih.gov/books/NBK220493/pdf/Bookshelf_NBK220493.pdf
- (McKeown & Brown, 1955):
“At first sight a list of developments in medicine during the eighteenth century seems impressive. It includes expansion of hospital, dispensary and midwifery services; notable changes in medical education; advances in understanding of physiology and morbid anatomy; and introduction of the first example of effective protective therapy (inoculation against smallpox). It is scarcely surprising that Griffith, like most other writers, should have concluded that these changes contributed materially to the health of the people. This conclusion derives from failure to distinguish clearly between the interests of the doctor and the interests of the patient, a common error in the interpretation of medical history. From the point of view of a student or practitioner of medicine, increased knowledge of anatomy, physiology and morbid anatomy are naturally regarded as important professional advances, as indeed they are. But from the point of view of the patient, none of these changes has any practical significance until such time as they contribute to preservation of health or recovery from illness. It is because there is often a substantial interval of time between acquisition of new knowledge and the possibility of any demonstrable benefit to the patient, that we cannot accept changes in medical education and institutions as evidence of the immediate effectiveness of medical effort. To arrive at a reliable opinion we must look closely at the work of doctors during the eighteenth century, and enquire whether in the light of modern knowledge it seems likely to have contributed to the health of their patients.
To sum up. Three possible causes of a reduction of mOitality from infectious disease are considered: specific medical therapy; changes in the balance between the virulence of the infective organism and its host; and improvements in the environment. Reasons have been given previously for rejecting the first cause, and it is suggested that although there have undoubtedly been changes in the character of individual infections, it is unreasonable to attribute to this alone the progressive decline in mortality from infections as a whole, after many centuries in which mortality remained high. Improvements in the environment are therefore regarded as intrinsically the most acceptable explanation of the decline of mortality in the late eighteenth and nineteenth centuries.
It is well recognised that in the late nineteenth century living conditions improved in ways which quite certainly influenced the course of the infectious diseases. Although there is no equally good evidence that living conditions improved in the last quarter of the eighteenth century-in some respects they probably deteriorated-it is quite conceivable that there was a general advance in the standard of living in consequence of the economic developments of the period. It is noted that whether we accept the birth rate or the death rate as the more important influence on the rise of the population, the conclusion that conditions improved in the late eighteenth century must follow rejection of the effectiveness of medical effort.”
McKeown, T., & Brown, R. G. (1955). Medical evidence related to English population changes in the eighteenth century. Population studies, 9(2), 119-141. DOI: 10.1080/00324728.1955.10404688. https://doi.org/10.1080/00324728.1955.10404688
- (McKinlay & McKinlay, 1977):
“Certainly, from the evidence considered here, only poliomyelitis appears to have had a noticeably changed death rate subsequent to intervention. Even if it were assumed that this change was entirely due to the vaccines, then only about one percent of the decline following interventions for the diseases considered here (column d of Table 1) could be attributed to medical measures. Rather more conservatively, if we attribute some of the subsequent fall in the death rates for pneumonia, influenza, whooping cough, and diphtheria to medical measures, then perhaps 3.5 percent of the fall in the overall death rate can be explained through medical intervention in the major infectious diseases considered here. Indeed, given that it is precisely for these diseases that medicine claims most success in lowering mortality, 3.5 percent probably represents a reasonable upper-limit estimate of the total contribution of medical measures to the decline in mortality in the United States since 1900. […] the introduction of specific medical measures and/or the expansion of medical services are generally not responsible for most of the modern decline in mortality.”
McKinlay, J. B., & McKinlay, S. M. (1977). The questionable contribution of medical measures to the decline of mortality in the United States in the twentieth century. The milbank memorial Fund Quarterly. health and Society, 405-428. DOI: 10.2307/3349539. https://doi.org/10.2307/3349539
- (Mersha et al., 2024):
“Although the [Dengvaxia] vaccine initially seemed to provide reasonable protection against dengue-related hospitalization after 2 years (89,2% overall and 72,6% in children of all ages), the risk of severe dengue was found to be higher in vaccinated children who were seronegative at baseline”
Aynekulu Mersha, D. G., van der Sterren, I., van Leeuwen, L. P. M., Langerak, T., Hakim, M. S., Martina, B., … & van Gorp, E. C. M. (2024). The role of antibody-dependent enhancement in dengue vaccination. Tropical Diseases, Travel Medicine and Vaccines, 10(1), 22. DOI: 10.1186/s40794-024-00231-2. https://doi.org/10.1186/s40794-024-00231-2 ; Recommended: https://link.springer.com/content/pdf/10.1186/s40794-024-00231-2.pdf
- (Mersha et al., 2024b):
“ADE is an immune-pathological phenomenon associated with increased disease severity in multiple viral infections. […] Viral infections where ADE is seen include several Flaviviruses, Coronaviruses, Ebola, HIV, RSV, measles and influenza, with dengue virus (DENV) being the most prominent example.”
Aynekulu Mersha, D. G., van der Sterren, I., van Leeuwen, L. P. M., Langerak, T., Hakim, M. S., Martina, B., … & van Gorp, E. C. M. (2024). The role of antibody-dependent enhancement in dengue vaccination. Tropical Diseases, Travel Medicine and Vaccines, 10(1), 22. DOI: 10.1186/s40794-024-00231-2. https://doi.org/10.1186/s40794-024-00231-2 ; Recommended: https://link.springer.com/content/pdf/10.1186/s40794-024-00231-2.pdf
- (Miller, 2021):
“If the 2605 deaths which occurred within 60 days of vaccination were randomly distributed throughout this interval, one would expect 43.42 deaths per day or 304 per week. The excess of deaths on the day of vaccination (43 were expected/440 occurred), within 3 days post-vaccination (130 were expected/1512 occurred), and in the first week post-vaccination (304 were expected/2041 occurred) were all statistically significant (p < 0.00001).
review of the medical literature substantiates a link between vaccines and sudden unexplained infant deaths. Several theories regarding the pathogenic mechanism behind these fatal events have been proposed, including the role of inflammatory cytokines as neuromodulators in the infant medulla preceding an abnormal response to the accumulation of carbon dioxide; fatal disorganization of respiratory control induced by adjuvants that cross the blood-brain barrier; and biochemical or synergistic toxicity due to multiple vaccines administered concurrently.”
Miller, N. Z. (2021). Vaccines and sudden infant death: An analysis of the VAERS database 1990–2019 and review of the medical literature. Toxicology Reports, 8, 1324-1335. DOI: 10.1016/j.toxrep.2021.06.020. https://doi.org/10.1016/j.toxrep.2021.06.020
- (Miller & Goldman, 2011):
“Linear regression analysis of unweighted mean IMRs showed a high statistically significant correlation between increasing number of vaccine doses and increasing infant mortality rates, with r = 0.992 (p = 0.0009).”
Miller, N. Z., & Goldman, G. S. (2011). Infant mortality rates regressed against number of vaccine doses routinely given: Is there a biochemical or synergistic toxicity?. Human & Experimental Toxicology, 30(9), 1420-1428. DOI: 10.1177/0960327111407644. https://doi.org/10.1177/0960327111407644 ; Recommended: https://journals.sagepub.com/doi/reader/10.1177/0960327111407644
- (Miller & Goldman, 2023):
“There are statistically significant positive correlations between mortality rates of developed nations and the number of early childhood vaccine doses that are routinely given.”
Miller, N. Z., & Goldman, G. S. (2023). Neonatal, infant, and under age five vaccine doses routinely given in developed nations and their association with mortality rates. Cureus, 15(7). DOI: 10.7759/cureus.42194. https://doi.org/10.7759/cureus.42194 ; Recommended: https://assets.cureus.com/uploads/original_article/pdf/164423/20230720-7739-2r4wve.pdf
- (Miller et al., 1972):
“Diphtheria toxoid helps prevent symptomatic disease but does not prevent the carrier state nor stop the spread of infection.”
Miller, L. W., Older, J. J., Drake, J., & Zimmerman, S. (1972). Diphtheria immunization: effect upon carriers and the control of outbreaks. American journal of diseases of children, 123(3), 197-199. DOI: 10.1001/archpedi.1972.02110090067004. https://doi.org/10.1001/archpedi.1972.02110090067004
- (Miller et al., 1981):
“The relative risk of neurological illness in immunised as compared with unimmunised children (table VIII) was 2.4 (p<0.001). Twenty of the notified children (2.0%) and 18 controls (0.9%) had been immunised within 72 hours, with a relative risk of 2.6 (p < 0.01). Thus significantly more notified infants than controls had been immunised during the seven days and especially the 72 hours before the onset of the neurological disorders. When the analysis was confined to children apparently previously normal, the differences were larger and the relative risks higher. […] Most of the children notified to the study with serious neurological disorders had not received any inununisation within seven days of their first symptoms, and their illnesses must be attributed to other causes. There was, however, a statistically significant association with diphtheria, tetanus, and pertussis vaccine given within the previous seven days, and especially within 72 hours. Similar analysis for diphtheria and tetanus vaccine showed a slight excess of immunisation within seven days but the relative risk was not significant at the 5% level.”
Miller, D. L., Ross, E. M., Alderslade, R., Bellman, M. H., & Rawson, N. S. (1981). Pertussis immunisation and serious acute neurological illness in children. Br Med J (Clin Res Ed), 282(6276), 1595-1599. DOI: 10.1136/bmj.282.6276.1595. https://doi.org/10.1136/bmj.282.6276.1595 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1505512/pdf/bmjcred00658-0031.pdf
- (Mitchell et al., 2010):
Mitchell, E. A., Elder, D. E., & Zuccollo, J. (2010). Simultaneous sudden unexpected death in infancy of twins: case report. International journal of legal medicine, 124(6), 631-635. DOI: 10.1007/s00414-009-0407-y. https://doi.org/10.1007/s00414-009-0407-y ; Recommended: https://link.springer.com/content/pdf/10.1007/s00414-009-0407-y.pdf
- (Mogensen et al., 2017):
“DTP was associated with 5-fold higher mortality than being unvaccinated. No prospective study has shown beneficial survival effects of DTP. Unfortunately, DTP is the most widely used vaccine, and the proportion who receives DTP3 is used globally as an indicator of the performance of national vaccination programs. It should be of concern that the effect of routine vaccinations on all-cause mortality was not tested in randomized trials. All currently available evidence suggests that DTP vaccine may kill more children from other causes than it saves from diphtheria, tetanus or pertussis. Though a vaccine protects children against the target disease it may simultaneously increase susceptibility to unrelated infections. […] In this situation the children were allocated by birthday to receive vaccines early or late and the ‘unvaccinated‘ were therefore not frail children. […] The negative effect of DTP was much worse in this natural experiment than has been reported in previous studies of DTP. This is presumably due to the “unvaccinated” control children in previous studies having been a frail subgroup too frail to get vaccinated. Previous studies have not been able to compare DTP-vaccinated children with “normal” controls. Hence, most previous studies have probably underestimated the negative effect of DTP. […] It should be of concern that the effect of routine vaccinations on all-cause mortality was not tested in randomized trials. All currently available evidence suggests that DTP vaccine may kill more children from other causes than it saves from diphtheria, tetanus or pertussis. Though a vaccine protects children against the target disease it may simultaneously increase susceptibility to unrelated infections.”
Mogensen, S. W., Andersen, A., Rodrigues, A., Benn, C. S., & Aaby, P. (2017). The introduction of diphtheria-tetanus-pertussis and oral polio vaccine among young infants in an urban African community: a natural experiment. EBioMedicine, 17, 192-198. DOI: 10.1016/j.ebiom.2017.01.041. https://doi.org/10.1016/j.ebiom.2017.01.041 ; Recommended: https://www.thelancet.com/action/showPdf?pii=S2352-3964%2817%2930046-4
- (Murphy et al., 2001):
Murphy, T. V., Gargiullo, P. M., Massoudi, M. S., Nelson, D. B., Jumaan, A. O., Okoro, C. A., … & Livingood, J. R. (2001). Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine, 344(8), 564-572. DOI: 10.1056/NEJM200102223440804. https://doi.org/10.1056/NEJM200102223440804 ; Recommended: https://www.nejm.org/doi/pdf/10.1056/NEJM200102223440804
- (National Childhood Vaccine Injury Act of 1986, 1986):
“Provides that no vaccine manufacturer shall be liable in a civil action for damages arising from a vaccine-related injury or death: (1) resulting from unavoidable side effects”
H.R.5546 - 99th Congress (1985-1986): National Childhood Vaccine Injury Act of 1986. (1986). https://www.congress.gov/bill/99th-congress/house-bill/5546
- (Nohynek et al., 2012):
Nohynek, H., Jokinen, J., Partinen, M., Vaarala, O., Kirjavainen, T., Sundman, J., … & Kilpi, T. (2012). AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS one, 7(3), e33536. DOI: 10.1371/journal.pone.0033536. https://doi.org/10.1371/journal.pone.0033536 ; Recommended: https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0033536&type=printable
- (O'Leary et al., 2024):
“under value-based care models, pediatricians may receive a significant part of their payments based on performance metrics, one of which is completion of childhood and adolescent immunizations.”
O‘Leary, S. T., Opel, D. J., Cataldi, J. R., & Hackell, J. M. (2024). Strategies for improving vaccine communication and uptake. Pediatrics, 153(3), e2023065483. DOI: 10.1542/peds.2023-065483. https://doi.org/10.1542/peds.2023-065483 ; Recommended: https://publications.aap.org/pediatrics/article-pdf/153/3/e2023065483/1601274/peds.2023-065483.pdf
- (Offit et al., 2002):
“vaccine-specific antibody responses and rates of vaccine-associated adverse reactions of children with mild or moderate illnesses are comparable to those of healthy children […] Studies on the diversity of antigen receptors indicate that the immune system has the capacity to respond to extremely large numbers of antigens.[…] Although we now give children more vaccines, the actual number of antigens they receive has declined. […] the short-lived immunosuppression caused by certain vaccines does not result in an increased risk of infections with other pathogens soon after vaccination. Vaccinated children are not at greater risk of subsequent infections with other pathogens than unvaccinated children. On the contrary, in Germany, a study of 496 vaccinated and unvaccinated children found that children who received immunizations against diphtheria, pertussis, tetanus, Hib, and polio within the first 3 months of life had fewer infections with vaccine-related and -unrelated pathogens than the nonvaccinated group.”
Offit, P. A., Quarles, J., Gerber, M. A., Hackett, C. J., Marcuse, E. K., Kollman, T. R., … & Landry, S. (2002). Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system?. Pediatrics, 109(1), 124-129. DOI: 10.1542/peds.109.1.124. https://doi.org/10.1542/peds.109.1.124
- (Offit & Jew, 2003):
Offit, P. A., & Jew, R. K. (2003). Addressing parents’ concerns: do vaccines contain harmful preservatives, adjuvants, additives, or residuals?. Pediatrics, 112(6), 1394-1397. DOI: 10.1542/peds.112.6.1394. https://doi.org/10.1542/peds.112.6.1394
- (Offit, 2005):
“The agency’s scientists found that two production pools made by Cutter Laboratories (accounting for 120,000 doses) contained live poliovirus. Among the children who had received vaccine from these pools, abortive polio (characterized by headache, stiff neck, fever, and muscle weakness) developed in 40,000; 51 were permanently paralyzed; and 5 died. Cutter’s vaccine also started a polio epidemic: 113 people in the children’s families or communities were paralyzed, and 5 died.”
Offit, P. A. (2005). The Cutter incident, 50 years later. New England Journal of Medicine, 352(14), 1411-1412. DOI: 10.1056/NEJMp048180. https://doi.org/10.1056/NEJMp048180
- (Offit, 2008):
“Unfortunately, in recent years the [Vaccine Injury Compensation Program] VICP seems to have turned its back on science. In 2005, Margaret Althen successfully claimed that a tetanus vaccine had caused her optic neuritis. Although there was no evidence to support her claim, the VICP ruled that if a petitioner proposed a biologically plausible mechanism by which a vaccine could cause harm, as well as a logical sequence of cause and effect, an award should be granted. The door opened by this and other rulings allowed petitioners to claim successfully that the MMR vaccine caused fibromyalgia and epilepsy, the hepatitis B vaccine caused Guillain–Barré syndrome and chronic demyelinating polyneuropathy, and the Hib vaccine caused transverse myelitis.
No case, however, represented a greater deviation from the VICP’s original standards than that of Dorothy Werderitsh, who in 2006 successfully claimed that a hepatitis B vaccine had caused her multiple sclerosis. By the time of the ruling, several studies had shown that hepatitis B vaccine neither caused nor exacerbated the disease, and the Institute of Medicine had concluded that “evidence favors rejection of a causal relationship between hepatitis B vaccine and multiple sclerosis.” But the VICP was less impressed with the scientific literature than it was with an expert’s proposal of a mechanism by which hepatitis B vaccine could induce autoimmunity (an ironic conclusion, given that Dorothy Werderitsh never had a detectable immune response to the vaccine).[…]Like the Werderitsh decision, the VICP’s concession to Hannah Poling was poorly reasoned.”
Offit, P. A. (2008). Vaccines and autism revisited—the Hannah Poling case. New England Journal of Medicine, 358(20), 2089-2091. DOI: 10.1056/NEJMp0802904. https://doi.org/10.1056/NEJMp0802904
- (Ohmit et al., 2013):
“There was no evidence that vaccination prevented household transmission once influenza was introduced; adults were at particular risk despite vaccination.”
Ohmit, S. E., Petrie, J. G., Malosh, R. E., Cowling, B. J., Thompson, M. G., Shay, D. K., & Monto, A. S. (2013). Influenza vaccine effectiveness in the community and the household. Clinical infectious diseases, 56(10), 1363-1369. DOI: 10.1093/cid/cit060. https://doi.org/10.1093/cid/cit060 ; Recommended: https://academic.oup.com/cid/article-pdf/56/10/1363/1129430/cit060.pdf
- (Onakpoya et al., 2016):
“The interval between launch date and reports of adverse drug reactions has shortened over the past few decades […] However, withdrawal of products following reports of suspected adverse reactions, sufficiently serious to warrant withdrawal, has not improved consistently over the last 60 years.”
Onakpoya, I. J., Heneghan, C. J., & Aronson, J. K. (2016). Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC medicine, 14, 1-11. DOI: 10.1186/s12916-016-0553-2. https://doi.org/10.1186/s12916-016-0553-2 ; Recommended: https://link.springer.com/content/pdf/10.1186/s12916-016-0553-2.pdf
- (Orenstein & Ahmed, 2017):
“Vaccines not only provide individual protection for those persons who are vaccinated, they can also provide community protection by reducing the spread of disease within a population (Fig. 1). Person-to-person infection is spread when a transmitting case comes in contact with a susceptible person. If the transmitting case only comes in contact with immune individuals, then the infection does not spread beyond the index case and is rapidly controlled within the population.
Interestingly, this chain of human-to-human transmission can be interrupted, even if there is not 100% immunity, because transmitting cases do not have infinite contacts; this is referred to as “herd immunity” or “community protection,” and is an important benefit of vaccination.
Mathematical modelers can estimate on average how many persons the typical transmitting case is capable of infecting if all of the contacts were susceptible (i.e., a population of 100% susceptibility). This number is known as R0, or the basic reproductive number. The immunity threshold needed within the population for terminating transmission can be calculated in percent as (R0 − 1)/R0 × 100 and is a guide to setting immunity levels and vaccination coverage targets for various diseases (8). For example, measles is one of the most contagious of vaccine-preventable diseases, with an estimated immunity threshold of 92–94%. In contrast, the protection threshold for rubella is estimated at 83–85%. Thus, eliminating rubella transmission is easier than measles, and when there are gaps in immunization coverage leading to accumulation of susceptibles, measles is often the first vaccine-preventable disease identified. Because of community protection induced by vaccines, persons who cannot be vaccinated (e.g., have contraindications or are younger than the age for whom vaccines are recommended), as well as persons who fail to make an adequate immune response to the vaccine (although most vaccines are highly effective, they are not 100% effective), can be protected indirectly because they are not exposed (Fig. 1). Thus, for most vaccines, achieving high levels of coverage is important not only for individual protection but in preventing disease in vulnerable populations that cannot be directly protected by vaccination. This provides the rationale for interventions to achieve high population immunity, such as removing barriers that may prevent access to vaccines (e.g., providing recommended vaccines without cost), as well as mandates for immunization requirements for attending school (9).”
Orenstein, W. A., & Ahmed, R. (2017). Simply put: Vaccination saves lives. Proceedings of the National Academy of Sciences, 114(16), 4031-4033. DOI: 10.1073/pnas.1704507114. https://doi.org/10.1073/pnas.1704507114 ; Recommended: https://www.pnas.org/doi/epdf/10.1073/pnas.1704507114
- (Ozawa et al., 2016):
“Exhibit 2: Estimated Return On Investment (ROI), Economic Benefits, And Costs Of Immunization Programs For 10 Antigens, By Country Group, 2011–20 […] Broader economic benefits […] $1.12–$1.96 trillion”
Ozawa, S., Clark, S., Portnoy, A., Grewal, S., Brenzel, L., & Walker, D. G. (2016). Return on investment from childhood immunization in low-and middle-income countries, 2011–20. Health Affairs, 35(2), 199-207. DOI: 10.1377/hlthaff.2015.1086. https://doi.org/10.1377/hlthaff.2015.1086
- (Papania et al., 2017):
Papania, M. J., Zehrung, D., & Jarrahian, C. (2017). Technologies to improve immunization. Plotkin‘s Vaccines, 1320. DOI: 10.1016/B978-0-323-35761-6.00068-7. https://doi.org/10.1016/B978-0-323-35761-6.00068-7 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC7152424/pdf/main.pdf
- (Parrino & Graham, 2006):
Parrino, J., & Graham, B. S. (2006). Smallpox vaccines: Past, present, and future. Journal of allergy and clinical immunology, 118(6), 1320-1326. DOI: 10.1016/j.jaci.2006.09.037. https://doi.org/10.1016/j.jaci.2006.09.037 ; Recommended: https://www.jacionline.org/article/S0091-6749(06)02013-6/pdf
- (Persson et al., 2014):
Persson, I., Granath, F., Askling, J., Ludvigsson, J. F., Olsson, T., & Feltelius, N. (2014). Risks of neurological and immune‐related diseases, including narcolepsy, after vaccination with P andemrix: a population‐and registry‐based cohort study with over 2 years of follow‐up. Journal of internal medicine, 275(2), 172-190. DOI: 10.1111/joim.12150. https://doi.org/10.1111/joim.12150 ; Recommended: https://onlinelibrary.wiley.com/doi/epdf/10.1111/joim.12150
- (Poland & Kennedy, 2022):
“Vaccines have known side effects, both local and systemic, that may be caused by various mechanisms.”
Poland, G. A., & Kennedy, R. B. (2022). Vaccine safety in an era of novel vaccines: a proposed research agenda. Nature Reviews Immunology, 22(4), 203-204. DOI: 10.1038/s41577-022-00695-3. https://doi.org/10.1038/s41577-022-00695-3 ; Recommended: https://www.nature.com/articles/s41577-022-00695-3.pdf
- (Prygiel et al., 2022):
“The pertussis vaccine was licensed in 1914. The same year, the mixtures of diphtheria toxin and antitoxin were put into use.”
Prygiel, M., Mosiej, E., Gorska, P., & Zasada, A. A. (2022). Diphtheria–tetanus–pertussis vaccine: Past, current & future. Future Microbiology, 17(3), 185-197. DOI: 10.2217/fmb-2021-0167. https://doi.org/10.2217/fmb-2021-0167
- (Puliyel & Naikm, 2018):
Puliyel, J., & Naik, P. (2018). Revised World Health Organization (WHO)’s causality assessment of adverse events following immunization—a critique. F1000Research, 7, 243. DOI: 10.12688/f1000research.13694.2. https://doi.org/10.12688/f1000research.13694.2
- (Rauh & Schmidt, 1965):
Rauh, L. W., & Schmidt, R. (2000). Measles immunization with killed virus vaccine. Serum antibody titers and experience with exposure to measles epidemic. 1965. Bulletin of the World Health Organization, 78(2), 226. DOI: 10.1001/archpedi.1965.02090020234007. https://doi.org/10.1001/archpedi.1965.02090020234007 ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC2560693/pdf/10743294.pdf
- (Rawlins, 1988):
“there is clear evidence that only a small proportion (rarely exceeding 10 to 15%) of even severe reactions are reported”
Rawlins, M. D. (1988). Spontaneous reporting of adverse drug reactions. I: the data. British journal of clinical pharmacology, 26(1), 1. DOI: 10.1111/j.1365-2125.1988.tb03356.x. https://doi.org/10.1111/j.1365-2125.1988.tb03356.x ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC1386492/pdf/brjclinpharm00097-0007.pdf
- (Rawlins, 1995):
“estimates of the completeness of reporting [of adverse drug reactions] suggest that it is rare for more than 10% of serious reactions to be reported; and that reporting is rarely better than 2-4% for non-serious reactions. At least part of the problem is the relatively poor reporting by hospital doctors who contribute only a third of reports despite the fact that serious reactions are most likely to present in hospital.”
Rawlins, M. D. (1995). Pharmacovigilance: paradise lost, regained or postponed? The William Withering Lecture 1994. Journal of the Royal College of Physicians of London, 29(1), 41. https://pubmed.ncbi.nlm.nih.gov/7738878/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC5401160/pdf/jrcollphyslond90369-0041.pdf
- (Rawlins, 1995b):
“For example, in the early 1970s, annual whooping cough vaccination rates were consistently around 60-70% (Fig 1). In 1974, however, stories began to appear in the medical literature of a possible association between pertussis vaccination and permanent neurological damage. The story was quickly adopted by the lay media and, as a consequence, vaccination rates fell. The numbers of cases rose. Only during the 1980s, as accumulating evidence confirmed that pertussis vaccination was not causally associated with irreversible brain damage, did vaccination rates rise again and the incidence of whooping cough fall. The failure to undertake effective pharmacovigilance of whooping cough vaccines in the 1960s was directly responsible for a public health disaster in the 1970s and early 1980s.”
Rawlins, M. D. (1995). Pharmacovigilance: paradise lost, regained or postponed? The William Withering Lecture 1994. Journal of the Royal College of Physicians of London, 29(1), 41. https://pubmed.ncbi.nlm.nih.gov/7738878/ ; Recommended: https://pmc.ncbi.nlm.nih.gov/articles/PMC5401160/pdf/jrcollphyslond90369-0041.pdf
- (Remschmidt et al., 2015):
Remschmidt, C., Wichmann, O., & Harder, T. (2015). Frequency and impact of confounding by indication and healthy vaccinee bias in observational studies assessing influenza vaccine effectiveness: a systematic review. BMC infectious diseases, 15(1), 429. DOI: 10.1186/s12879-015-1154-y. https://doi.org/10.1186/s12879-015-1154-y ; Recommended: https://link.springer.com/content/pdf/10.1186/s12879-015-1154-y.pdf
- (Rid et al., 2014):
“Placebo use in vaccine trials is clearly acceptable when (a) no efficacious and safe vaccine exists and (b) the vaccine under consideration is intended to benefit the population in which the vaccine is to be tested. In this situation, a placebo-controlled trial addresses the locally relevant question regarding the extent to which the new vaccine is better than nothing, and participants in the placebo arm of the trial are not deprived of the clinical benefits of an existing efficacious vaccine.”
Rid, A., Saxena, A., Baqui, A. H., Bhan, A., Bines, J., Bouesseau, M. C., … & Smith, P. G. (2014). Placebo use in vaccine trials: recommendations of a WHO expert panel. Vaccine, 32(37), 4708-4712. DOI: 10.1016/j.vaccine.2014.04.022. https://doi.org/10.1016/j.vaccine.2014.04.022 ; Recommended: https://www.sciencedirect.com/science/article/pii/S0264410X14005374/pdfft?md5=45bd31a3ff56daa0d01cca20d87c9b99&pid=1-s2.0-S0264410X14005374-main.pdf
- (Rid et al., 2014b):
“Placebo use in vaccine trials is clearly unacceptable when (a) a highly efficacious and safe vaccine exists and is currently accessible in the public health system of the country in which the trial is planned and (b) the risks to participants of delaying or foregoing the available vaccine cannot be adequately minimized or mitigated (e.g. by providing counselling and education on behavioural disease prevention strategies, or ensuring adequate treatment for the condition under study to prevent serious harm).”
Rid, A., Saxena, A., Baqui, A. H., Bhan, A., Bines, J., Bouesseau, M. C., … & Smith, P. G. (2014b). Placebo use in vaccine trials: recommendations of a WHO expert panel. Vaccine, 32(37), 4708-4712. DOI: 10.1016/j.vaccine.2014.04.022. https://doi.org/10.1016/j.vaccine.2014.04.022 ; Recommended: https://www.sciencedirect.com/science/article/pii/S0264410X14005374/pdfft?md5=45bd31a3ff56daa0d01cca20d87c9b99&pid=1-s2.0-S0264410X14005374-main.pdf
- (Riemersma et al., 2022):
“infectious SARS-CoV-2 is found at similar titers in vaccinated and unvaccinated persons”
Riemersma, K. K., Haddock III, L. A., Wilson, N. A., Minor, N., Eickhoff, J., Grogan, B. E., … & Grande, K. M. (2022). Shedding of infectious SARS-CoV-2 despite vaccination. PLoS Pathogens, 18(9), e1010876. DOI: 10.1371/journal.ppat.1010876. https://doi.org/10.1371/journal.ppat.1010876 ; Recommended: https://journals.plos.org/plospathogens/article/file?id=10.1371/journal.ppat.1010876&type=printable
- (Roberts, 1987):
Roberts, S. C. (1987). Vaccination and cot deaths in perspective. Archives of Disease in Childhood, 62(7), 754-759. DOI: 10.1136/adc.62.7.754. https://doi.org/10.1136/adc.62.7.754 ; Recommended: https://adc.bmj.com/content/archdischild/62/7/754.full.pdf
- (Rodrigues & Plotkin, 2020):
“The most significant impact of vaccines has been to prevent morbidity and mortality from serious infections that disproportionately affect children. Vaccines are estimated to prevent almost six million deaths/year and to save 386 million life years and 96 million disability-adjusted life years (DALYs) globally (Ehreth, 2003).”
Rodrigues, C. M., & Plotkin, S. A. (2020). Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology, 11, 1526. DOI: 10.3389/fmicb.2020.01526. https://doi.org/10.3389/fmicb.2020.01526 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.01526/pdf
- (Rodrigues & Plotkin, 2020b):
“At the time of writing, the only infectious disease that has been eradicated in humans by vaccination is smallpox. This disease had afflicted humans for millenia, with the earliest evidence found in Egyptian mummies from 1000 BC (Geddes, 2006).”
Rodrigues, C. M., & Plotkin, S. A. (2020). Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology, 11, 1526. DOI: 10.3389/fmicb.2020.01526. https://doi.org/10.3389/fmicb.2020.01526 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.01526/pdf
- (Rodrigues & Plotkin, 2020c):
“Two polio vaccines, the inactivated polio vaccine (IPV) and the live-attenuated oral polio vaccine (OPV) became available in 1955 and 1963, respectively (Plotkin, 2014), both able to protect against all three wild types of polio virus. Both vaccines have been used globally, with live-attenuated OPV much cheaper and easier to administer but carrying the risk of causing circulating vaccine-derived poliovirus (cVDPV) owing to back-mutation and re-acquisition of neurovirulence.”
Rodrigues, C. M., & Plotkin, S. A. (2020). Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology, 11, 1526. DOI: 10.3389/fmicb.2020.01526. https://doi.org/10.3389/fmicb.2020.01526 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.01526/pdf
- (Rodrigues & Plotkin, 2020d):
“The overriding health benefit perceived by most vaccine recipients is their personal, direct, protection. The added value of vaccination, on a population level, is the potential to generate herd immunity. Where a sufficiently high proportion of the population are vaccinated, transmission of the infecting agent is halted thereby protecting the unvaccinated, who may be those too young, too vulnerable, or too immunosuppressed to receive vaccines.
Herd (population) immunity requires high levels of vaccine uptake, to limit the number of unvaccinated people and the opportunity for pathogen transmission between them. The proportion of a given population required to induce herd immunity through vaccination is lower for the bacterial infections and conjugate polysaccharide vaccines, as their basic reproductive number (R0) is lower than viral infections like measles, varicella or polio […] TABLE 2 Vaccines with the potential to induce herd immunity”
Rodrigues, C. M., & Plotkin, S. A. (2020). Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology, 11, 1526. DOI: 10.3389/fmicb.2020.01526. https://doi.org/10.3389/fmicb.2020.01526 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.01526/pdf
- (Rodrigues & Plotkin, 2020e):
“Globally, the provision of vaccines is more challenging in many low- and middle- income countries (LMIC), as evidenced by the failure to make the EPI vaccines available to every child by 1990, irrespective of setting (Keja et al., 1988). […] Nevertheless, there has been a decrease in the global burden of diseases caused by vaccine-preventable pathogens (Figure 3) enabling healthier lives for many millions of children.”
Rodrigues, C. M., & Plotkin, S. A. (2020). Impact of vaccines; health, economic and social perspectives. Frontiers in microbiology, 11, 1526. DOI: 10.3389/fmicb.2020.01526. https://doi.org/10.3389/fmicb.2020.01526 ; Recommended: https://public-pages-files-2025.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.01526/pdf
- (Roush et al., 2007):
Roush, S. W., Murphy, T. V., & Vaccine-Preventable Disease Table Working Group. (2007). Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. Jama, 298(18), 2155-2163. DOI: 10.1001/jama.298.18.2155. https://doi.org/10.1001/jama.298.18.2155
- (Sala-i-Martin, 2006):
“We estimate the World Distribution of Income by integrating individual income distributions for 138 countries between 1970 and 2000. Country distributions are constructed by combining national accounts GDP per capita to anchor the mean with survey data to pin down the dispersion. Poverty rates and head counts are reported for four specific poverty lines. Rates in 2000 were between one-third and one-half of what they were in 1970 for all four lines. There were between 250 and 500 million fewer poor in 2000 than in 1970. We estimate eight indexes of income inequality implied by our world distribution of income. All of them show reductions in global inequality during the 1980s and 1990s.”
Sala-i-Martin, X. (2006). The world distribution of income: falling poverty and… convergence, period. The quarterly journal of economics, 121(2), 351-397. DOI: 10.1162/qjec.2006.121.2.351. https://doi.org/10.1162/qjec.2006.121.2.351
- (Salmon et al., 2024):
Salmon, D. A., Orenstein, W. A., Plotkin, S. A., & Chen, R. T. (2024). Funding postauthorization vaccine-safety science. The New England journal of medicine, 391(2), 102-105. DOI: 10.1056/nejmp2402379. https://doi.org/10.1056/nejmp2402379
- (Salmon et al., 2024b):
“Progress in vaccine-safety science has understandably been slow — often depending on epidemiologic evidence that is delayed or is inadequate to support causal conclusions and on an understanding of biologic mechanisms that is incomplete — which has adversely affected vaccine acceptance. […] Postauthorization studies are needed to fully characterize the safety profile of a new vaccine, since prelicensure clinical trials have limited sample sizes, follow up durations, and population heterogeneity. […] Over the past two decades, many new vaccines have been introduced for children and for vulnerable populations such as pregnant women and older adults. However, aside from emergency appropriations for the H1N1 influenza and Covid-19 pandemics, the budget for vaccine-safety monitoring at the CDC (which is responsible for the majority of U.S. federal efforts) has remained stagnant during this period, at about $20 million per year. Although these resources have been used efficiently, this inadequate level of funding has adversely affected the speed and completeness of the science.”
Salmon, D. A., Orenstein, W. A., Plotkin, S. A., & Chen, R. T. (2024). Funding postauthorization vaccine-safety science. The New England journal of medicine, 391(2), 102-105. DOI: 10.1056/nejmp2402379. https://doi.org/10.1056/nejmp2402379
- (Schonberger et al., 1979):
Schonberger, L. B., Bregman, D. J., Sullivan-Bolyai, J. Z., Keenlyside, R. A., Ziegler, D. W., Retailliau, H. F., … & Bryan, J. A. (1979). Guillain-Barré syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. American journal of epidemiology, 110(2), 105-123. DOI: 10.1093/oxfordjournals.aje.a112795. https://doi.org/10.1093/oxfordjournals.aje.a112795
- (Scrimshaw, 2003):
Scrimshaw, N. S. (2003). Historical concepts of interactions, synergism and antagonism between nutrition and infection. The Journal of nutrition, 133(1), 316S-321S. DOI: 10.1093/jn/133.1.316S. https://doi.org/10.1093/jn/133.1.316S
- (Shattock et al., 2024):
Shattock, A. J., Johnson, H. C., Sim, S. Y., Carter, A., Lambach, P., Hutubessy, R. C., … & Bar-Zeev, N. (2024). Contribution of vaccination to improved survival and health: modelling 50 years of the Expanded Programme on Immunization. The Lancet, 403(10441), 2307-2316. DOI: 10.1016/S0140-6736(24)00850-X. https://doi.org/10.1016/S0140-6736(24)00850-X ; Recommended: https://www.thelancet.com/action/showPdf?pii=S0140-6736%2824%2900850-X
- (Steelman Anything, 2025):
Steelman Anything. (2025). Analysis of currently approved vaccines in the U.S. from https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states [Data set] [Unpublished raw data]. https://steelmananything.com/assets/data/approved_vaccines_united_states.xlsx
- (Tramer et al., 1998):
“In some trials placebos were omitted on ethical grounds. This is illogical because studies destined to produce unreliable results should themselves be considered unethical.”
Tramèr, M. R., Reynolds, D. J. M., Moore, R. A., & McQuay, H. J. (1998). When placebo controlled trials are essential and equivalence trials are inadequate. Bmj, 317(7162), 875-880. DOI: 10.1136/bmj.317.7162.875. https://doi.org/10.1136/bmj.317.7162.875
- (Traversa et al., 2011):
Traversa, G., Spila-Alegiani, S., Bianchi, C., Ciofi degli Atti, M., Frova, L., Massari, M., … & Hera Study Group. (2011). Sudden unexpected deaths and vaccinations during the first two years of life in Italy: A case series study. PLoS One, 6(1), e16363. DOI: 10.1371/journal.pone.0016363. https://doi.org/10.1371/journal.pone.0016363 ; Recommended: https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0016363&type=printable
- (U.S. CDC, 1999):
U.S. CDC. (1999). Withdrawal of rotavirus vaccine recommendation. MMWR. Morbidity and mortality weekly report, 48(43), 1007. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4843a5.htm
- (U.S. CDC, 1999b):
U.S. CDC (1999). Impact of vaccines universally recommended for children–United States, 1990-1998. MMWR. Morbidity and mortality weekly report, 48(12), 243-248. https://www.cdc.gov/mmwr/pdf/wk/mm4812.pdf
- (U.S. CDC, 2022):
“Continued monitoring has identified nine deaths causally associated with J&J/Janssen COVID-19 vaccination.”
U.S. CDC (2022). Selected adverse events reported after COVID-19 vaccination. Retrieved July, 2022, from https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
- (U.S. CDC, 2024):
U.S. CDC (2024). Historical Vaccine Concerns. Retrieved November, 2025, from https://www.cdc.gov/vaccine-safety/historical-concerns/index.html
- (U.S. CDC, 2024b):
“As with any medicine, there is a very remote chance of a vaccine causing a severe allergic reaction, other serious injury, or death.”
U.S. CDC (2024). Possible Side Effects from Vaccines. Retrieved November, 2025, from https://www.cdc.gov/vaccines/basics/possible-side-effects.html
- (U.S. CDC, 2024c):
“Inactivated poliovirus vaccine (IPV) […] protects people from polio disease but does not stop transmission of the virus.”
U.S. CDC (2024). Polio Disease and Poliovirus Containment. Retrieved November, 2025, from https://www.cdc.gov/poliovirus-containment/diseaseandvirus/index.html
- (U.S. Committee on Government Reform, 2000):
“Former FDA Commissioner David A. Kessler has estimated that VAERS reports currently represent only a fraction of the serious adverse events.”
The Vaccine Injury Compenstation Program Addressing Needs and Improving Practices: U.S. Committee on Government Reform, 106th Congress (2000). https://www.congress.gov/106/crpt/hrpt977/CRPT-106hrpt977.pdf
- (U.S. FDA, 1978):
“Procedure:” ([multiple])
U.S. FDA. (1978). Combined Live Measles Virus Vaccine (Moraten Line-ATTENUVAX) Jeryl Lynn Mumps Virus Vaccine (MUMPSVAX) RA 27/3 Rubella Virus Vaccine. Retrieved April, 2025, from https://www.sirillp.com/wp-content/uploads/2023/07/MMRII-FOIA.pdf ; Recommended: https://www.sirillp.com/wp-content/uploads/2023/07/MMRII-FOIA.pdf#page=30
- (U.S. FDA, 1998):
Package Insert: “ADVERSE REACTIONS”
U.S. FDA. (1998). Liquid PedvaxHIB® [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)]. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/haemophilus-b-conjugate-vaccine-meningococcal-protein-conjugate
- (U.S. FDA, 1998b):
“During a three-day period following primary vaccinatio”
U.S. FDA. (1998). Liquid PedvaxHIB® [Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)]. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/haemophilus-b-conjugate-vaccine-meningococcal-protein-conjugate
- (U.S. FDA, 2001):
“A useful approach to the assessment of assay sensitivity in active control trials and in placebo-controlled trials is the three-arm trial, including both placebo and a known active treatment, a trial design with several advantages. Such a trial measures effect size (test drug versus placebo) and allows comparison of test drug and active control in a setting where assay sensitivity is established by the active control versus placebo comparison.”
U.S. FDA. (2001). E10 Choice of Control Group and Related Issues in Clinical Trials. Retrieved April, 2025, from https://www.fda.gov/media/71349/download ; Recommended: https://www.fda.gov/media/71349/download#page=18
- (U.S. FDA, 2001b):
“An active control (positive control) trial is one in which an investigational drug is compared with a known active drug.”
U.S. FDA. (2001b). E10 Choice of Control Group and Related Issues in Clinical Trials. Retrieved April, 2025, from https://www.fda.gov/media/71349/download ; Recommended: https://www.fda.gov/media/71349/download#page=27
- (U.S. FDA, 2002):
Package Insert: “Results of Clinical Evaluations”
U.S. FDA. (2002). Prevnar 7: Pneumococcal 7-valent Conjugate Vaccine (Diphtheria CRM197 Protein). Retrieved October, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar
- (U.S. FDA, 2006):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2006). GARDASIL® [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant] Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil
- (U.S. FDA, 2007):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2007). Influenza Virus Vaccine, H5N1 Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-virus-vaccine-h5n1-national-stockpile
- (U.S. FDA, 2009):
Package Insert: “CLINICAL STUDIES”
U.S. FDA. (2009). TICE BCG attenuated, live culture preparation of the Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bovis. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/tice-bcg
- (U.S. FDA, 2009b):
Package Insert: “Clinical Studies Experience”
U.S. FDA. (2009b). CERVARIX [Human Papillomavirus Bivalent (Types 16 and 18) Vaccine, Recombinant] Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/cervarix
- (U.S. FDA, 2009c):
Package Insert: “Safety Experience from Clinical Studies”
U.S. FDA. (2009c). Influenza A (H1N1) 2009 Monovalent Vaccine Manufactured by CSL Limited Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent-vaccine-csl-limited
- (U.S. FDA, 2009d):
Package Insert: “Adverse Reactions in Clinical Trials”
U.S. FDA. (2009d). Influenza A (H1N1) 2009 Monovalent Vaccine Live, Intranasal Manufactured by MedImmune, LLC Intranasal Spray. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent-vaccine-medimmune-llc
- (U.S. FDA, 2009e):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2009e). Influenza A (H1N1) 2009 Monovalent Vaccine Manufactured by ID Biomedical Corporation of Quebec (IDB) Distributed by GlaxoSmithKline (GSK) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent-vaccine-id-biomedical-corporation-quebec
- (U.S. FDA, 2009f):
Package Insert: “Clinical Trial Experience”
U.S. FDA. (2009f). Influenza A (H1N1) 2009 Monovalent Vaccine Manufactured by Novartis Vaccines and Diagnostics Ltd. Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent-vaccine-novartis-vaccines-and-diagnostics-limited
- (U.S. FDA, 2009g):
Package Insert: “Clinical Trial Experience”
U.S. FDA. (2009g). Influenza A (H1N1) 2009 Monovalent Vaccine Manufactured by Sanofi Pasteur Inc. Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent-vaccine-sanofi-pasteur-inc
- (U.S. FDA, 2013):
Package Insert: “Adverse Reactions”
U.S. FDA. (2013). Vivotif® Typhoid Vaccine Live Oral Ty21a. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/vivotif
- (U.S. FDA, 2015):
Package Insert: “receive saline”
U.S. FDA. (2015). BIOTHRAX® (Anthrax Vaccine Adsorbed) Suspension for Intramuscular or Subcutaneous Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/biothrax
- (U.S. FDA, 2016):
“The active control must be a drug whose effect is well-defined. The most obvious choice is a single drug for which historical placebo-controlled trials are available”
U.S. FDA. (2016). Non-Inferiority Clinical Trials to Establish Effectiveness Guidance for Industry. Retrieved November, 2025, from https://www.fda.gov/media/78504/download
- (U.S. FDA, 2017):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2017). Prevnar 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-13
- (U.S. FDA, 2017b):
Package Insert: “6-month follow-up”
U.S. FDA. (2017b). Prevnar 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-13
- (U.S. FDA, 2017c):
Package Insert: “There were 3 (0.063%) deaths among Prevnar 13 recipients, and 1 (0.036%) death in Prevnar recipients, all as a result of sudden infant death syndrome (SIDS). These SIDS rates are consistent with published age specific background rates of SIDS from the year 2000.”
U.S. FDA. (2017c). Prevnar 13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-13
- (U.S. FDA, 2017d):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2017d). FLUVIRIN® (Influenza Virus Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluvirin
- (U.S. FDA, 2018):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2018). RECOMBIVAX HB® Hepatitis B Vaccine (Recombinant) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/recombivax-hb
- (U.S. FDA, 2018b):
Package Insert: “monitored for 5 days after each dose”
U.S. FDA. (2018b). RECOMBIVAX HB® Hepatitis B Vaccine (Recombinant) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/recombivax-hb
- (U.S. FDA, 2018c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2018c). Menactra®, Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/menactra
- (U.S. FDA, 2018d):
Package Insert: “CLINICAL PHARMACOLOGY”
U.S. FDA. (2018d). BCG VACCINE (TICE® strain). Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/bcg-vaccine
- (U.S. FDA, 2018e):
Package Insert: “Data from Clinical Trials”
U.S. FDA. (2018e). IXIARO (Japanese Encephalitis Vaccine, Inactivated, Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/ixiaro
- (U.S. FDA, 2018f):
Package Insert: “Data from Clinical Trials”
U.S. FDA. (2018f). Tetanus and Diphtheria Toxoids Adsorbed TDVAX. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/tdvax
- (U.S. FDA, 2019):
Package Insert: “placebo (lactose tablets)”
U.S. FDA. (2019). Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Enteric Coated Tablets for Oral Administration. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/adenovirus-type-4-and-type-7-vaccine-live-oral
- (U.S. FDA, 2019b):
Package Insert: “Data from Clinical Studies”
U.S. FDA. (2019b). YF-VAX Yellow Fever Vaccine. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/yf-vax
- (U.S. FDA, 2019c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2019c). ZOSTAVAX® (Zoster Vaccine Live) Suspension for subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/zostavax
- (U.S. FDA, 2020):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2020). AGRIFLU, Influenza Virus Vaccine Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/agriflu
- (U.S. FDA, 2020b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2020b). TYPHOID VI POLYSACCHARIDE VACCINE TYPHIM VI. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/typhim-vi
- (U.S. FDA, 2020c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2020c). VAQTA® (Hepatitis A Vaccine, Inactivated) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/vaqta
- (U.S. FDA, 2020d):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2020d). RotaTeq (Rotavirus Vaccine, Live, Oral, Pentavalent) Oral Solution. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/rotateq
- (U.S. FDA, 2021):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2021). TICOVAC (Tick-Borne Encephalitis Vaccine), Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/ticovac
- (U.S. FDA, 2021b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2021b). PNEUMOVAX® 23 (pneumococcal vaccine polyvalent) Sterile, Liquid Vaccine for Intramuscular or Subcutaneous Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/pneumovax-23-pneumococcal-vaccine-polyvalent
- (U.S. FDA, 2022):
Package Insert: “ADVERSE REACTIONS”
U.S. FDA. (2022). IPOL - Poliovirus Vaccine Inactivated (Monkey Kidney Cell). Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/ipol-poliovirus-vaccine-inactivated-monkey-kidney-cell
- (U.S. FDA, 2022b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022b). TENIVAC (Tetanus and Diphtheria Toxoids Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/tenivac
- (U.S. FDA, 2022c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022c). PRIORIX (Measles, Mumps, and Rubella Vaccine, Live), suspension for subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/priorix
- (U.S. FDA, 2022d):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022d). ROTARIX (Rotavirus Vaccine, Live, Oral) Suspension, for oral use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/rotarix
- (U.S. FDA, 2022e):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022e). DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/daptacel
- (U.S. FDA, 2022f):
Package Insert: “through 6 months following the last dose”
U.S. FDA. (2022f). DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/daptacel
- (U.S. FDA, 2022g):
Package Insert: “There was one death due to aspiration 222 days post-vaccination in a subject with ependymoma”
U.S. FDA. (2022g). DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/daptacel
- (U.S. FDA, 2022h):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022h). Quadracel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/quadracel
- (U.S. FDA, 2022i):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022i). Pentacel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) Vaccine Suspension for Intramuscular Injection (inactivated poliovirus component grown in MRC-5 cells). Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/pentacel
- (U.S. FDA, 2022j):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022j). Pentacel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) Vaccine Suspension for Intramuscular Injection (inactivated poliovirus component grown in Vero cells). Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/pentacel
- (U.S. FDA, 2022k):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2022k). ActHIB [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)] Solution for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/acthib
- (U.S. FDA, 2022y):
“Myocarditis/pericarditis, in particular in the first week following Dose 2, is a known risk associated with the Pfizer-BioNTech COVID-19 Vaccine and is greatest among adolescent males 16-17 years of age compared with both younger and older age groups.” (Page 65)
U.S. FDA (2022y). Emergency use authorization (EUA) for an unapproved product review memorandum. Retrieved July, 2022, from https://www.fda.gov/media/159393/download#page=65
- (U.S. FDA, 2023):
Package Insert: “ADVERSE REACTIONS”
U.S. FDA. (2023). HIBERIX [Haemophilus b Conjugate Vaccine (Tetanus Toxoid Conjugate)] for injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/hiberix
- (U.S. FDA, 2023b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023b). Adacel (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed), Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/adacel
- (U.S. FDA, 2023c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023c). CYFENDUS™ (Anthrax Vaccine Adsorbed, Adjuvanted) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/cyfendus
- (U.S. FDA, 2023d):
Package Insert: “saline placebo”
U.S. FDA. (2023d). PEDIARIX [Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Hepatitis B (Recombinant) and Inactivated Poliovirus Vaccine], Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/pediarix
- (U.S. FDA, 2023e):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023e). TWINRIX [Hepatitis A & Hepatitis B (Recombinant) Vaccine] injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/twinrix
- (U.S. FDA, 2023f):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023f). IXCHIQ (Chikungunya Vaccine, Live) Solution for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/ixchiq
- (U.S. FDA, 2023g):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023g). FLUAD QUADRIVALENT (Influenza Vaccine, Adjuvanted) Injectable Emulsion for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/fluad-quadrivalent
- (U.S. FDA, 2023h):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023h). FLUCELVAX QUADRIVALENT (Influenza Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flucelvax-quadrivalent
- (U.S. FDA, 2023i):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023i). FLULAVAL QUADRIVALENT (Influenza Vaccine) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flulaval-quadrivalent
- (U.S. FDA, 2023j):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023j). Flublok Quadrivalent (Influenza Vaccine), Sterile Solution for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flublok-quadrivalent
- (U.S. FDA, 2023k):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023k). FluMist® Quadrivalent (Influenza Vaccine Live, Intranasal) Intranasal Spray. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist-quadrivalent
- (U.S. FDA, 2023l):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023l). FLUARIX QUADRIVALENT (Influenza Vaccine) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluarix-quadrivalent
- (U.S. FDA, 2023m):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023m). DENGVAXIA (Dengue Tetravalent Vaccine, Live) Suspension for Subcutaneous Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/dengvaxia
- (U.S. FDA, 2023n):
Package Insert: “DENGVAXIA is not approved for use in individuals younger than 6 years of age. These individuals, regardless of previous infection by dengue virus, are at increased risk of severe and hospitalized dengue disease following vaccination with DENGVAXIA and subsequent infection with any dengue virus serotype. DENGVAXIA is not approved for use in individuals not previously infected by any dengue virus serotype or for whom this information is unknown. Those not previously infected are at increased risk for severe dengue disease when vaccinated and subsequently infected with dengue virus. […] The safety and effectiveness of DENGVAXIA have not been established in individuals living in dengue nonendemic areas who travel to dengue endemic areas.”
U.S. FDA. (2023n). DENGVAXIA (Dengue Tetravalent Vaccine, Live) Suspension for Subcutaneous Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/dengvaxia
- (U.S. FDA, 2023o):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023o). INFANRIX (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/infanrix
- (U.S. FDA, 2023p):
Package Insert: “monitored for unsolicited adverse events that occurred within 28 days following vaccination”
U.S. FDA. (2023p). INFANRIX (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/infanrix
- (U.S. FDA, 2023q):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023q). KINRIX (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/kinrix
- (U.S. FDA, 2023r):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023r). VAXELIS® (Diphtheria and Tetanus Toxoids and Acellular Pertussis Inactivated Poliovirus, Haemophilus b Conjugate and Hepatitis B Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaxelis
- (U.S. FDA, 2023s):
Package Insert: “or saline placebo”
U.S. FDA. (2023s). ERVEBO® (Ebola Zaire Vaccine, Live) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/ervebo
- (U.S. FDA, 2023t):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023t). HAVRIX (Hepatitis A Vaccine) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/havrix
- (U.S. FDA, 2023u):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023u). ENGERIX-B Hepatitis B Vaccine (Recombinant) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/engerix-b
- (U.S. FDA, 2023v):
Package Insert: “monitored for 4 days post-administration”
U.S. FDA. (2023v). ENGERIX-B Hepatitis B Vaccine (Recombinant) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/engerix-b
- (U.S. FDA, 2023w):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023w). VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxneuvance
- (U.S. FDA, 2023x):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023x). PREVNAR 20® (Pneumococcal 20-valent Conjugate Vaccine), suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20
- (U.S. FDA, 2023y):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023y). BOOSTRIX (Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine, Adsorbed) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/boostrix
- (U.S. FDA, 2023z):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2023z). VARIVAX Varicella Virus Vaccine Live Suspension for intramuscular or subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/varivax-refrigerated-and-frozen-formulations
- (U.S. FDA, 2024):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024). BEXSERO (Meningococcal Group B Vaccine) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/bexsero
- (U.S. FDA, 2024b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024b). FLUAD (Influenza Vaccine, Adjuvanted) Injectable Emulsion for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluad
- (U.S. FDA, 2024c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024c). AFLURIA QUADRIVALENT, Influenza Vaccine Suspension for Intramuscular Injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/afluria-quadrivalent-afluria-quadrivalent-southern-hemisphere
- (U.S. FDA, 2024d):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024d). FLULAVAL (Influenza Vaccine) Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flulaval
- (U.S. FDA, 2024e):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024e). FluMist (Influenza Vaccine Live, Intranasal) Nasal Spray, for intranasal use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flumist
- (U.S. FDA, 2024f):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024f). FLUARIX (Influenza Vaccine) Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluarix
- (U.S. FDA, 2024g):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024g). Flublok (Influenza Vaccine) Injection for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flublok
- (U.S. FDA, 2024h):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024h). FLUCELVAX (Influenza Vaccine) Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/flucelvax
- (U.S. FDA, 2024i):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024i). M-M-R® II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for intramuscular or subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/measles-mumps-and-rubella-virus-vaccine-live
- (U.S. FDA, 2024j):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024j). PENBRAYA® (Meningococcal Groups A, B, C, W, and Y Vaccine), suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/penbraya
- (U.S. FDA, 2024k):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024k). CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) Injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/capvaxive
- (U.S. FDA, 2024l):
Package Insert: “Clinical Studies”
U.S. FDA. (2024l). IMOVAX® Rabies vaccine. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/imovax-rabies
- (U.S. FDA, 2024m):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024m). RabAvert Rabies Vaccine. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/rabavert
- (U.S. FDA, 2024n):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024n). MRESVIATM (Respiratory Syncytial Virus Vaccine) Injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/mresvia
- (U.S. FDA, 2024o):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024o). JYNNEOS (Smallpox and Mpox Vaccine, Live, Non-replicating) suspension for subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/jynneos
- (U.S. FDA, 2024p):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024p). VAXCHORA® (Cholera Vaccine, Live, Oral) Suspension for Oral Administration. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxchora
- (U.S. FDA, 2024q):
Package Insert: “ADVERSE REACTIONS”
U.S. FDA. (2024q). COMIRNATY® (COVID-19 Vaccine, mRNA) suspension for injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/comirnaty
- (U.S. FDA, 2024r):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024r). HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted] injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/heplisav-b
- (U.S. FDA, 2024s):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024s). AREPANRIX (Influenza A [H5N1] Virus Monovalent Vaccine, Adjuvanted) injectable emulsion, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/arepanrix
- (U.S. FDA, 2024t):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024t). AUDENZ (Influenza A (H5N1) Monovalent Vaccine, Adjuvanted) injectable emulsion for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/audenz
- (U.S. FDA, 2024u):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024u). Fluzone (Influenza Vaccine) Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluzone-and-fluzone-high-dose
- (U.S. FDA, 2024v):
Package Insert: “placebo concomitantly (N=205) at separate injection sites”
U.S. FDA. (2024v). ProQuad Measles, Mumps, Rubella and Varicella Virus Vaccine Live Suspension for intramuscular or subcutaneous injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/proquad
- (U.S. FDA, 2024w):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024w). MENVEO [Meningococcal (Groups A, C, Y, and W-135) Oligosaccharide Diphtheria CRM 197 Conjugate Vaccine] solution for injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/menveo
- (U.S. FDA, 2024x):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024x). TRUMENBA® (Meningococcal Group B Vaccine) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/trumenba
- (U.S. FDA, 2024y):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2024y). MenQuadfi, Meningococcal (Groups A, C, Y, W) Conjugate Vaccine Injection for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/menquadfi
- (U.S. FDA, 2025):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025). VIMKUNYA™ (Chikungunya Vaccine, Recombinant) injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vimkunya
- (U.S. FDA, 2025b):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025b). Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/afluria-afluria-southern-hemisphere
- (U.S. FDA, 2025c):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025c). Fluzone (Influenza Vaccine) Injectable Suspension, for Intramuscular Use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/fluzone-quadrivalent-fluzone-high-dose-quadrivalent-fluzone-intradermal-quadrivalent-fluzone
- (U.S. FDA, 2025d):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025d). PENMENVY (Meningococcal Groups A, B, C, W, and Y Vaccine) for injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/penmenvy
- (U.S. FDA, 2025e):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025e). ABRYSVO® (Respiratory Syncytial Virus Vaccine) for injection, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/abrysvo
- (U.S. FDA, 2025f):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025f). AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) for injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/arexvy
- (U.S. FDA, 2025g):
Package Insert: “CLINICAL STUDIES”
U.S. FDA. (2025g). ACAM2000® [Smallpox and Mpox (Vaccinia) Vaccine, Live] For scarification suspension, for percutaneous use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/acam2000
- (U.S. FDA, 2025h):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025h). SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) for injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/shingrix
- (U.S. FDA, 2025i):
Package Insert: “ADVERSE REACTIONS”
U.S. FDA. (2025i). SPIKEVAX (COVID-19 Vaccine, mRNA) Injectable suspension, for intramuscular use. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/spikevax
- (U.S. FDA, 2025j):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025j). GARDASIL 9 (Human Papillomavirus 9-valent Vaccine, Recombinant) Suspension for intramuscular injection. Retrieved April, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9
- (U.S. FDA, 2025k):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025k). MNEXSPIKE (COVID-19 Vaccine, mRNA) injectable suspension, for intramuscular use. Retrieved July, 2025, from https://www.fda.gov/vaccines-blood-biologics/mnexspike
- (U.S. FDA, 2025l):
Package Insert: “Clinical Trials Experience”
U.S. FDA. (2025l). NUVAXOVID® (COVID-19 Vaccine, Adjuvanted) injectable suspension, for intramuscular use. Retrieved July, 2025, from https://www.fda.gov/vaccines-blood-biologics/vaccines/nuvaxovid
- (U.S. Health Resources & Services Administration, 2025):
“Concession: HHS concludes that a petition should be compensated based on a thorough review and analysis of the evidence, including medical records and the scientific and medical literature. The HHS review concludes that the petitioner is entitled to compensation, including a determination either that it is more likely than not that the vaccine caused the injury or the evidence supports fulfillment of the criteria of the Vaccine Injury Table. The Court also determines that the petition should be compensated.”
U.S. Public Health Service. (2025). National Vaccine Injury Compensation Program Data Report. Retrieved November, 2025, from https://www.hrsa.gov/sites/default/files/hrsa/vicp/vicp-stats-09-01-25.pdf
- (U.S. Health Resources & Services Administration, 2025b):
“If the first symptom of these injuries and/or conditions occurs within the specified time periods and the injury meets the definition included in the Table, it is presumed that the vaccine caused the injury or condition unless another cause is proven.”
U.S. Public Health Service. (2025). National Vaccine Injury Compensation Program Data Report. Retrieved November, 2025, from https://www.hrsa.gov/vaccine-compensation/covered-vaccines
- (U.S. Public Health Service, 1950):
U.S. Public Health Service. (1950). Vital Statistics of the United States 1948 Part II Natality and Mortality Data for the United States Tabulated by Place of Residence. https://www.cdc.gov/nchs/data/vsus/vsus_1948_2.pdf#page=244
- (Valiant et al., 2022):
Valiant, W. G., Cai, K., & Vallone, P. M. (2022). A history of adventitious agent contamination and the current methods to detect and remove them from pharmaceutical products. Biologicals, 80, 6-17. DOI: 10.1016/j.biologicals.2022.10.002. https://doi.org/10.1016/j.biologicals.2022.10.002
- (Vanderslott et al., 2019):
Vanderslott, S., Phillips, M. T., Pitzer, V. E., & Kirchhelle, C. (2019). Water and filth: reevaluating the first era of sanitary typhoid intervention (1840–1940). Clinical Infectious Diseases, 69(Supplement_5), S377-S384. DOI: 10.1093/cid/ciz610. https://doi.org/10.1093/cid/ciz610 ; Recommended: https://academic.oup.com/cid/article-pdf/69/Supplement_5/S377/30325999/ciz610.pdf
- (Varricchio et al., 2004):
“Enhanced passive surveillance detected rare cases of viscerotropic and neurotropic disease after yellow fever vaccine, allowing them to be more fully characterized.”
Varricchio, F., Iskander, J., Destefano, F., Ball, R., Pless, R., Braun, M. M., & Chen, R. T. (2004). Understanding vaccine safety information from the vaccine adverse event reporting system. The Pediatric infectious disease journal, 23(4), 287-294. https://journals.lww.com/pidj/abstract/2004/04000/understanding_vaccine_safety_information_from_the.2.aspx
- (Varricchio et al., 2004b):
“During the recent resumption of smallpox vaccination, cases of myopericarditis and ischemic cardiac events among smallpox vaccine recipients have been reported to VAERS. Although a causal association between smallpox vaccine and cardiac events has not been definitively established, the Advisory Committee on Immunization Practices now recommends that persons with physician-diagnosed cardiac disease and risk factors be excluded from smallpox vaccination.”
Varricchio, F., Iskander, J., Destefano, F., Ball, R., Pless, R., Braun, M. M., & Chen, R. T. (2004). Understanding vaccine safety information from the vaccine adverse event reporting system. The Pediatric infectious disease journal, 23(4), 287-294. https://journals.lww.com/pidj/abstract/2004/04000/understanding_vaccine_safety_information_from_the.2.aspx
- (Varricchio et al., 2004c):
“VAERS data contain strong biases. […] Reports of special interest, including all deaths and hospitalizations, are followed up by epidemiologists at CDC or FDA. Follow-up often yields important information. For example investigation of reported deaths determined that the cause of death was significantly different from what originally was stated on the VAERS report in 24% of the cases.4 In one case the vaccinee had in fact not died. […] There appears to be a widespread, but mistaken, perception that all adverse reactions are reported to VAERS.42 Only selected adverse events, as specified in the Reportable Events Table, are required by law to be reported by vaccine providers.”
Varricchio, F., Iskander, J., Destefano, F., Ball, R., Pless, R., Braun, M. M., & Chen, R. T. (2004). Understanding vaccine safety information from the vaccine adverse event reporting system. The Pediatric infectious disease journal, 23(4), 287-294. https://journals.lww.com/pidj/abstract/2004/04000/understanding_vaccine_safety_information_from_the.2.aspx
- (Von Kries et al., 2005):
Von Kries, R., Toschke, A. M., Straßburger, K., Kundi, M., Kalies, H., Nennstiel, U., … & Giani, G. (2005). Sudden and unexpected deaths after the administration of hexavalent vaccines (diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B, Haemophilius influenzae type b): is there a signal?. European journal of pediatrics, 164(2), 61-69. DOI: 10.1007/s00431-004-1594-7. https://doi.org/10.1007/s00431-004-1594-7 ; Recommended: https://link.springer.com/content/pdf/10.1007/s00431-004-1594-7.pdf
- (Warfel et al., 2014):
“Although pertussis resurgence is not completely understood, we hypothesize that current acellular pertussis (aP) vaccines fail to prevent colonization and transmission. To test our hypothesis, infant baboons were vaccinated at 2, 4, and 6 mo of age with aP or whole-cell pertussis (wP) vaccines and challenged with B. pertussis at 7 mo. Infection was followed by quantifying colonization in nasopharyngeal washes and monitoring leukocytosis and symptoms. Baboons vaccinated with aP were protected from severe pertussis-associated symptoms but not from colonization, did not clear the infection faster than naïve animals, and readily transmitted B. pertussis to unvaccinated contacts.Vaccination with wP induced a more rapid clearance compared with naïve and aP-vaccinated animals. By comparison, previously infected animals were not colonized upon secondary infection. Although all vaccinated and previously infected animals had robust serum antibody responses, we found key differences in T-cell immunity. Previously infected animals and wP-vaccinated animals possess strong B. pertussis-specific T helper 17 (Th17) memory and Th1 memory, whereas aP vaccination induced a Th1/Th2 response instead. The observation that aP, which induces an immune response mismatched to that induced by natural infection, fails to prevent colonization or transmission provides a plausible explanation for the resurgence of pertussis and suggests that optimal control of pertussis will require the development of improved vaccines.”
Warfel, J. M., Zimmerman, L. I., & Merkel, T. J. (2014). Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proceedings of the National Academy of Sciences, 111(2), 787-792. DOI: 10.1073/pnas.1314688110. https://doi.org/10.1073/pnas.1314688110 ; Recommended: https://www.pnas.org/doi/pdf/10.1073/pnas.1314688110
- (Warfel & Merkel, 2014):
Warfel, J. M., & Merkel, T. J. (2014). Reply to Domenech de Celles et al.: Infection and transmission of pertussis in the baboon model. Proceedings of the National Academy of Sciences, 111(7), E718-E718. DOI: 10.1073/pnas.1324074111. https://doi.org/10.1073/pnas.1324074111 ; Recommended: https://www.pnas.org/doi/epdf/10.1073/pnas.1324074111
- (Werne & Garrow, 1946):
Werne, J., & Garrow, I. (1946). Fatal anaphylactic shock: occurrence in identical twins following second injection of diphtheria toxoid and pertussis antigen. Journal of the American Medical Association, 131(9), 730-735. DOI: 10.1001/jama.1946.02870260014003. https://doi.org/10.1001/jama.1946.02870260014003
- (Whitney et al., 2014):
“Modeling estimated that, among children born during 1994– 2013, vaccination will prevent an estimated 322 million illnesses, 21 million hospitalizations, and 732,000 deaths over the course of their lifetimes, at a net savings of $295 billion in direct costs and $1.38 trillion in total societal costs. With support from the VFC program, immunization has been a highly effective tool for improving the health of U.S. children.”
Whitney, C. G., Zhou, F., Singleton, J., Schuchat, A., & Centers for Disease Control and Prevention (CDC). (2014). Benefits from immunization during the vaccines for children program era-United States, 1994-2013. MMWR Morb Mortal Wkly Rep, 63(16), 352-355. https://www.cdc.gov/mmwr/pdf/wk/mm6316.pdf
- (Wilcox, 1983):
Wilcox, C. S. (1983). Regulation of renal blood flow by plasma chloride. The Journal of clinical investigation, 71(3), 726-735. DOI: 10.1172/JCI110820. https://doi.org/10.1172/JCI110820 ; Recommended: https://www.jci.org/articles/view/110820/version/1/pdf/render.pdf
- (Williams & Feely, 1999):
Williams, D., & Feely, J. (1999). Underreporting of adverse drug reactions: attitudes of Irish doctors. Irish journal of medical science, 168, 257-261. DOI: 10.1007/BF02944353. https://doi.org/10.1007/BF02944353
- (Wilson, 1967):
“Diphteria […] Dallas, Texas, 1919 […] Among the 96 reactions, 10 were very severe and resulted in death
Tashkent 1926 […] Fourteen children received by accident diphtheria toxin instead of anatoxin. Eight of them died within a fortnight and four more within a month after suffering from polyneuritis
Medellín, Colombia, 1930 […] the Medellín disaster resulted from the use of pure toxin in place of a harmless diphtheria prophylactic […] Altogether 16 of the 48 children died
Italy 1933 […] several hundred infants and children in the provinces of Rovigo and Venezia became severely ill after injection with what was believed to be anatoxin. Over 30 of the children died
Kyoto, Japan, 1948 […] a toxic batch of alum-precipitated toxoid (APT) was responsible for illness in over 600 infants and children and for no fewer than 68 deaths
[… more examples excluded … ]
The inherent danger of all vaccination procedures should be a deterrent to their unnecessary or unjustifiable use. Vaccination is far too often employed, especially in the developing countries, to avoid the tedious, troublesome and sometimes expensive process of improving personal and environmental hygiene. Admittedly there are some diseases of which vaccination is the only effective means of control, but even so it should not be introduced for routine use without making reasonably sure that it can be carried out under conditions that will more or less guarantee its effectiveness. […] Once a vaccine has been introduced, with apparently good results, it becomes extremely difficult ever to find out its real value. Moral objections may be too strong to permit a properly controlled trial. So long as there is some reason to believe that the vaccine is of benefit, it is considered unjustifiable to protect only a proportion of the children in a field trial and expose the others to the risk of the disease. […] Vaccines, of one sort or another, have conferred immense benefit on mankind but, like aeroplanes and motor-cars, they have their dangers.”
Wilson, S. G. S. (1967). The hazards of immunization (p. 66). London: Athlone Press. https://scholar.google.com/scholar?q=Wilson+The+Hazards+of+Immunization
- (World Health Organization, 2014):
“The safety of a new vaccine is assessed in clinical trials before it can be licensed. (2014). Principles and considerations for adding a vaccine to a national immunization programme: from decision to implementation and monitoring. https://iris.who.int/handle/10665/111548 ; Recommended: https://iris.who.int/bitstream/handle/10665/111548/9789241506892_eng.pdf
- (World Health Organization, 2014b):
“[clinical] trials may not capture rare adverse events and thus post-marketing surveillance may be needed to further establish the vaccine‘s safety profile. Information on safety should be assessed carefully, weighing the risks against the benefit of the vaccine.”
World Health Organization. (2014). Principles and considerations for adding a vaccine to a national immunization programme: from decision to implementation and monitoring. https://iris.who.int/handle/10665/111548 ; Recommended: https://iris.who.int/bitstream/handle/10665/111548/9789241506892_eng.pdf
- (World Health Organization, 2023):
World Health Organization. (2023). Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19). World Health Organization. https://iris.who.int/server/api/core/bitstreams/705bb1fd-b79a-4cef-9682-dea662c294aa/content
- (Zhou et al., 2003):
“VAERS is a passive surveillance system: reports of events are voluntarily submitted by those who experience them, their caregivers, or others. Passive surveillance systems (e.g., VAERS) are subject to multiple limitations, including underreporting, reporting of temporal associations or unconfirmed diagnoses, and lack of denominator data and unbiased comparison groups. Because of these limitations, determining causal associations between vaccines and adverse events from VAERS reports is usually not possible.”
Zhou, W., Pool, V., Iskander, J. K., English-Bullard, R., Ball, R., Wise, R. P., … & Chen, R. T. (2003). Surveillance for safety after immunization: vaccine adverse event reporting system (VAERS)—United States, 1991–2001. MMWR Surveill Summ, 52(1), 1-24. https://www.cdc.gov/MMWR/Preview/mmwrhtml/ss5201a1.htm
- (Zhou et al., 2003b):
“During 1991–2001, VAERS received 128,717 reports, whereas >1.9 billion net doses of human vaccines were distributed […] Overall, 14.2% of all reports received in VAERS during 1991–2001 described serious adverse events (10) (Table 9). During 1991–2001, reports of deaths ranged from 1.4%–2.3%, and reports of life-threatening illness ranged from 1.4%–2.8% of all adverse event reports. […] The majority of these deaths were ultimately classified as sudden infant death syndrome (SIDS). Analysis of the age distribution and seasonality of infant deaths reported to VAERS indicated that they matched the age distribution and seasonality of SIDS; both peaked at aged 2–4 months and during the winter. […] The decrease in deaths reported to VAERS since 1992–1993 parallels the overall decrease in SIDS in the U.S. population since the implementation of the Back to Sleep campaign . Carefully controlled epidemiologic studies consistently have not found any association between SIDS and vaccines. FDA and the Institute of Medicine (IOM) reviewed 206 deaths reported to VAERS during 1990–1991. Only one death was believed to have resulted from a vaccine. The patient was a woman aged 28 years who died from Guillain-Barré syndrome after tetanus vaccination. IOM concluded that the majority of deaths reported to VAERS are temporally but not causally related to vaccination. A similar conclusion was reached regarding neonatal deaths temporally reported to VAERS in association with hepatitis B vaccination. […] The VAERS data should be interpreted with caution, because they describe events that occurred after vaccination but they do not necessarily imply that the events were caused by vaccination. […] FDA medical officers evaluate reporting rates of adverse events by lot, as needed, looking for unexpected patterns. During the 11 years, no lot needed to be recalled on this basis.”
Zhou, W., Pool, V., Iskander, J. K., English-Bullard, R., Ball, R., Wise, R. P., … & Chen, R. T. (2003). Surveillance for safety after immunization: vaccine adverse event reporting system (VAERS)—United States, 1991–2001. MMWR Surveill Summ, 52(1), 1-24. https://www.cdc.gov/MMWR/Preview/mmwrhtml/ss5201a1.htm
- (Zhou et al., 2005):
“Without a routine childhood vaccination program, the model estimated that in a cohort of 3,803,295 children, approximately 14.3 million cases of these diseases would occur, resulting in 33,564 deaths. These cases would result in direct costs of $12.3 billion and societal costs of $46.6 billion. Disease-associated costs with vaccination were $0.1 billion and $0.5 billion, respectively. The direct and societal costs of the routine childhood vaccination program with DTaP, Td, Hib, IPV, MMR, HB, and varicella vaccines were estimated to be $2.3 billion and $2.8 billion, respectively (Table 5). The calculated NPVs (net savings) of the routine childhood vaccination program from the direct cost and societal perspectives were $9.9 billion and $43.3 billion, respectively.”
Zhou, F., Santoli, J., Messonnier, M. L., Yusuf, H. R., Shefer, A., Chu, S. Y., … & Harpaz, R. (2005). Economic evaluation of the 7-vaccine routine childhood immunization schedule in the United States, 2001. Archives of pediatrics & adolescent medicine, 159(12), 1136-1144. DOI: 10.1001/archpedi.159.12.1136. https://doi.org/10.1001/archpedi.159.12.1136
- (Zhou et al., 2014):
“Analyses showed that routine childhood immunization with DTaP, Hib, IPV, MMR, HepB, VAR, PCV7, HepA, and Rota among the cohort of 4 261 494 will prevent ∼42 000 early deaths and 20 million cases of disease. The direct and societal costs averted by immunization program were $20.3 billion and $76.4 billion, respectively. The direct and societal costs of the routine childhood immunization program were estimated at $6.7 billion and $7.5 billion, respectively. The NPVs (net savings) of the routine childhood immunization program from the payers’ and societal perspectives were $13.5 billion and $68.8 billion, respectively”
Zhou, F., Shefer, A., Wenger, J., Messonnier, M., Wang, L. Y., Lopez, A., … & Rodewald, L. (2014). Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics, 133(4), 577-585. DOI: 10.1542/peds.2013-0698. https://doi.org/10.1542/peds.2013-0698
- (Zhou et al., 2024):
“Among approximately 117 million children born during 1994–2023, routine childhood vaccinations will have prevented approximately 508 million lifetime cases of illness, 32 million hospitalizations, and 1,129,000 deaths, at a net savings of $540 billion in direct costs and $2.7 trillion in societal costs.”
Zhou, F., Jatlaoui, T. C., Leidner, A. J., Carter, R. J., Dong, X., Santoli, J. M., … & Peacock, G. (2024). Health and Economic Benefits of Routine Childhood Immunizations in the Era of the Vaccines for Children Program-United States, 1994-2023. MMWR. Morbidity and mortality weekly report, 73(31), 682-685. DOI: 10.15585/mmwr.mm7331a2. https://doi.org/10.15585/mmwr.mm7331a2 ; Recommended: https://www.cdc.gov/mmwr/volumes/73/wr/pdfs/mm7331a2-H.pdf
- (Zhou et al., 2024b):
“TABLE 1. Estimated number of illnesses, hospitalizations, and deaths prevented by routine childhood immunization against selected vaccine-preventable diseases in 30 cohorts of children — United States, 1994–2023 […] Deaths prevented (x 1,000) […] Measles […] 85.0”
Zhou, F., Jatlaoui, T. C., Leidner, A. J., Carter, R. J., Dong, X., Santoli, J. M., … & Peacock, G. (2024). Health and Economic Benefits of Routine Childhood Immunizations in the Era of the Vaccines for Children Program-United States, 1994-2023. MMWR. Morbidity and mortality weekly report, 73(31), 682-685. DOI: 10.15585/mmwr.mm7331a2. https://doi.org/10.15585/mmwr.mm7331a2 ; Recommended: https://www.cdc.gov/mmwr/volumes/73/wr/pdfs/mm7331a2-H.pdf#page=3
- (Zimmermann & Curtis, 2019):
Zimmermann, P., & Curtis, N. (2019). Factors that influence the immune response to vaccination. Clinical microbiology reviews, 32(2), 10-1128. DOI: 10.1128/cmr.00084-18. https://doi.org/10.1128/cmr.00084-18 ; Recommended: https://journals.asm.org/doi/pdf/10.1128/cmr.00084-18
Share on
Twitter Facebook LinkedIn
Other topics: